By Leonardo Valdivia and Joaquín Letelier
Here is a report about sessions performed during the 8th European Zebrafish meeting held in Barcelona. The post is divided in different topics to make it easy to read. Of course we did not cover all sessions and tried to focus on talks related to developmental biology, but we have also included some interesting talks beyond the main topic of the Node. We hope you enjoy it! (as we did).
Advances in Imaging
Vikas Trivedi started the session using two-photon SPIM microscopy to image the developing heart. He showed that during its formation a variety of cells are present in the heart primordium, perform different behaviors and adopt diverse morphology; these cellular features are essential for proper development of this organ. Next, Andrea Bassi showed a new technology for blood flow observation based on optical projection tomography (OPT). By rotating the samples in 360˚ a 3D reconstruction of the vasculature is obtained, which is then used to measure speed and direction of blood cells with no need of fluorescent labeling. Later on, Peter Eimon nicely showed a method for phenotyping embryos using whole animal tomography. With this analysis it is possible to do high resolution reconstruction of the embryos and measure different phenotypic features. Taking advantage of this technique (named hyperdimensional in vivo phenotyping) he showed that drugs that generate craniofacial defects produce similar phenotypic “signatures”. Finally, Gopi Shah showed a 4-lens SPIM system to obtain 2D projection images of a whole embryo in real-time, which allowed deep analysis of early zebrafish development.
Morphogenesis and Organogenesis
One of the recurrent topics in the meeting was morphogenesis. Given the nice and attractive conditions of our favourite model organism, attending these talks was a pleasure for the eyes and the mind.
Caren Norden kicked-off the session with an excellent talk about non-apical dividing progenitors in the developing retina and how the retinal architecture have a role on the emergence of this mode of division; she showed that retinal ganglion cells loss shift mitosis to basal locations, repositioning divisions in the developing retina. Keeping on eye formation, Toshiaki Mochisuki next examined the development of the lens. He explored into the cell dynamic within this structure and how cells change their position over time: cell divisions are the driving force for the cell movement observed during lens development and cell contacts influence cell division orientation and differentiation.
After dealing with the eye, the topic moved into heart development. In this part, Emily Noël talked about heart looping; by mutant analysis and explant culture, she proposed a pathway for amplifying a tissue intrinsic looping mechanism. Later on, Nadia Mercader showed beautiful in vivo imaging of heart beating and how this movement controls morphogenesis of the epicardium. She also showed amazing optical tweezing experiments, which allowed her to move single cells in the pericardial cavity. We really enjoyed it.
Esteban Hoijman introduced the next change in focus with a nice in vivo analysis of the inner ear formation. By using a breathtaking live imaging approach he nicely described the hollowing of this structure (lumen expansion), and showed is mediated by an actomyosin mesh and hydrostatic pressure. In the next talk, Nikoalus Obholzer provided a framework for system-level studies of the ear formation creating a 4-dimensional cell-based atlas of the ear. Such a good contribution will be helpful for testing hypothesis about ear formation.
Live imaging kept us amazed in the last two talks. Rachel Verdon presented a detailed view of pronephros development and glomerular filtration, providing a good model for kidney organogenesis. Finally, physics met the early embryo and Amayra Hernandez-Vega showed a dissection of the cellular mechanics driving epiboly, based in hydrodynamics as gastrulation goes on.
The talks provided a very good overview on several systems and a good taste about how the morphogenetic processes can be analyzed from different points of view and using different tools.
Gene Regulation and Genomics
This session started with an encouraging talk by Kerstin Howe from Sanger Institute about new improvements in the zebrafish reference genome that will result in a new version (GRCz10) in early 2014. Next, Todd Townsend introduced a novel method to get gene expression profiles from different tissues during development. His group developed an elegant system (BLRP-Rpl-BirA technology) to capture entire polysomes from specific cells with high affinity. With those samples they can determine which genes are active in confined cells during any developmental stage. Later on, Gustavo Gómez performed ChIPseq and RNAseq analysis to dissect the downstream targets of Etsrp/Etv2, a transcription factor essential for induction of vascular lineages. This work will help unravel new pathways involved in the development of vasculature. Finally, Miler Lee showed how maternal factors are cleared and zygotic genes activated during the maternal to zygotic transition. Using a sequencing approach on wild type and morphant embryos, he is getting deeper in the mechanisms that govern this essential process early in development.
New Technologies for Gene Manipulation
During the last years an explosive growth of new technologies has made fish an outstanding model for gene manipulation. This session provided a good update of some new tricks and resources that all the researchers in our field should know.
In the first talk Peggy Jungke introduced a productive gene trap screen for driving the expression of inducible Cre recombinase in different tissues of the embryo. These new zebrafish lines will allow the researchers to use conditional alleles or lineage-tracing approaches (for example the recently published zebrabow!). You can search for driver lines in http://crezoo.crt-dresden.de/crezoo/.
Carole Gauron was next, who introduced new optogenetic tools to manipulate cellular parameters in single cells or restricted tissues. She was able to induce and report apoptosis with this approach, opening new opportunities for regenerative studies.
It is well known that one of the most popular advantages of the zebrafish is transgenesis and Christian Mosimann introduced a new method to do it. He adapted a system used for single insertions of DNA fragments in predefined locus in fly, a technology that was not available for fish. This opens the possibility for inserting your favourite fragment of DNA in a simple, reliable and rapid manner. It will be helpful to increase the number and position of these landing sites in different fish lines to perform transgenesis in a highly controlled fashion. We look forward to hear more about it.
The next two talks aimed to show and compare current efforts for generating knock-out fish models using different approaches. Raman Sood compared the efficiency of ZFN (Zinc Finger Nucleases) and TALENs (Transcription Activator Like Effector Nucleases), giving useful parameters to keep in mind, especially for labs that are currently starting (or thinking) to work with these powerful custom designed nucleases. The next talk was given by Gaurav Varshney who introduced a new method for mapping insertional elements in the zebrafish genome, from a collection of mutagenised fish via this approach. This work is framed into the ambitious project for mutating all coding element in the zebrafish genome. Gaurav updated the efforts for the insertional mutants and encourage the audience to visit http://research.nhgri.nih.gov/ZInC/ for searching mutants for particular genes. The database is frequently updated, so keep an eye on that.
Finally, Koichi Kawakami (the father of the well known tol2 approach in zebrafish) reported the efforts for a large-scale Gal4 gene trap screening. He showed quite a few examples of specific pattern for those lines, providing reliable driver tools for manipulating UAS driven transgenes. All the lines that he showed (and many others) are available in http://kawakami.lab.nig.ac.jp/ztrap/. Importantly, he encouraged the audience to visit his lab in Japan for screening fish and find new drivers. His group can provide travel grants, so contact Koichi if you are interested!
Brain and Neural Crest Development
In this plenary session one of the most interesting talks (only because we like eye development!) was by Henrik Boije, who showed elegant data relevant to the formation of the retina. Using knockdown and transplantation techniques, he is dissecting how intrinsic and extrinsic factors can alter cell fate during retinal maturation. Next, Myriam Roussigne introduced the role of fgf signaling in the left-right asymmetry of the brain. She based her studies on the parapineal organ, a small group of cells migrating collectively from the midline to the left side of the developing brain. She showed fgf signaling activation restricted to few parapineal cells on the left side could be a key step for migration.
Finally, Angela Nieto showed how newly generated neurons maintain cell-cycle factors in a silenced state, as re-enter to this process leads to neuronal death. She placed scratch2 as a key regulator: knocking down this gene induces postmitotic neurons to the re-enter the cell cycle. Normal expression of scratch2 gene maintains high p57 (cell cycle inhibitor) by downregulation of miR-25.
Stem Cells and Regeneration
Fish has great potential for providing clues about tissue regeneration, something that is missed (or nearly) in mammals. Therefore a lot of attention is paid to understand how we can improve this process taking lessons from our favourite model. In this session the focus were stem cells (which are obviously linked to the regenerative process) and how they are controlled and organised in different context in zebrafish.
In the first talk, Michell Reimer nicely took advantage of combined approaches in fish. He showed that dopamine controls development and regeneration of motor neurons; this idea came up after a drug screen performed in his lab, encouraging the multidisciplinary approaches for finding completely new data. Alessandro Brombin then showed how cells in the brain contribute to the optic tectum and torus semicircularis. By using 4-dimensional time-lapse imaging, he tracked single cells all over the way. Given the recently published zebrabow approach, we can anticipate that similar strategies will be now possible in multiple colours including also cell shape in the equation.
Many of us use finclip for genotyping fish lines and we know empirically that after a few time the missing tissue grows back; fin regeneration is a well-established (and simple) model for tissue regeneration, but importantly it can be also used as a model for vertebrate limb regeneration. Mate Varga explored into the cellular mechanisms controlling this process: he showed that autophagy is increased during caudal fin regeneration in adults and this event is required for carrying out this task. In the same line of research, Rita Mateus explored into how the regenerated fin tissue knows how much to grow; she involved the Hippo pathway and mechanical interaction between the cells as critical players. Both talks were complementary and generated interesting questions.
David Stachura introduced a change from the regeneration topic, performing clonal analysis of hematopoietic stem cells and progenitors (which is a nice model for studying developmental restriction). He presented a successful assay to isolate and enrich hematopoietic progenitors for studying differentiation. In the future, using different and new transgenic lines could nicely complement it.
The next two talks investigated aspects of stem cell biology in the zebrafish retina. Vincent Tropepe talked about a mutant with defects in the ciliary marginal zone, a region in the postembryonic retina that contains stem cells allowing life-long growth of the fish eye. Mutants had an enlarged stem cell niche with decreased differentiation, meaning that the process is blocked in the transition. In the zebrafish eye there is a second stem cell population that is more active during regeneration: the Müller glia. Using a protocol for killing photoreceptors in the adult retinae, David Hyde talked about signals that make Müller glia to re-enter in the cell cycle for contributing to regenerate lost cells. He showed a multistep process in which dying photoreceptors secrete factors that are received by Müller glia, which then also start to produce and secrete them for amplifying the signal. This results in enhanced proliferation that helps to repopulate lost photoreceptor cells.
Finally, Laure Bally-Cuif showed data from an emergent model for neural stem cell biology: the adult pallium. She showed how notch3 dependent signalling gates cell cycle entry and limits neural stem cell amplification, proposing this pathway as an essential node of control.
All the talks were fantastic and covered different aspects from a broad point of view, which were carefully chosen by the organizers.
Cancer
Scientist interested in cancer use different techniques to study the growth, development and migration of tumour cells, and analysis in living organisms is imperative. In recent years, zebrafish has become an attractive model for studying this disease.
In this special session we learned what is currently possible to do with our favourite model organism. The speakers showed a combination of transgenic lines, genetic and molecule screens, and cell biology.
In the first talk Thomas Look emphasised that neuroblastoma is the most common extra cranial solid tumour in children. His group developed different transgenic lines as accurate models for inducing these tumours, which will be ideal for elucidating the cellular mechanisms of the synergy between oncoproteins. The next talk was by Marina Mione who explored post-transcriptional gene regulation by micro-RNAs during cancer transformation, a process by which a normal cell begins behaving like a tumour cell. She focused on melanoma and also generated transgenic lines displaying highly invasive cancer.
One big bottleneck for treatment is when cancer cells become resistant to therapies. Shelly Sorrells addressed this issue analysing mutants for genes involved in the resistant to ionizing radiation. Next, Paul Essers presented data about a zebrafish mutant model for the von Hippe-Lindau tumor suppressor gene and how it is required for the DNA damage response pathway.
Finding new molecules to block metastasis is always a challenge in the fight against cancer and Viviana Gallardo presented efforts towards this end. She showed a rapid in vivo screening for collective and single cell migration inhibitors, using the lateral line primordium (collective migration) and immune cell system (single migration) as they share molecular signatures and migratory behaviour with tumour cells. Some identified compounds showed a strong specific inhibitory effect (including some natural extracts); those results are very promising for finding new pharmacological treatments.
The last talk was by Yi Feng. She introduced another transgenic tumour model and showed astonishing movies of in vivo interaction between leucocytes and tumour cells, with immune cells surrounding and “biting” malignant cells. Amazing.
A big round of applause end up the session and we probably got more questions than answers, which is a good sign of research going forward.
We have to say that the whole meeting was very good organized and all worked perfect. Moreover, key lectures by Sydney Brenner and Denis Duboule were fantastic, showing us how far biology can go.
Now Barcelona is gone, but we look forward to Oslo 2015!
Our best fishes!
 (14 votes)
(14 votes)
 Loading...
Loading...
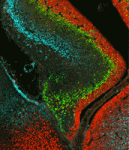 Mutation of neurofibromatosis 2 (NF2) results in nervous system tumours. Molecularly, Nf2 has diverse functions, regulating cell-cell junction formation and various signalling pathways, including the Hippo-Yap pathway. However, the roles of Nf2 in the nervous system, and how its loss promotes tumorigenesis, are poorly understood. Here (p. 3323), Xinwei Cao and co-workers analyse the consequences of Nf2 deletion in the dorsal telencephalon. Although the mutant mice are viable, they display significant brain malformations associated with neural progenitor cell (NPC) hyperproliferation. To determine how Nf2 limits NPC expansion, the authors performed a microarray analysis and found many known targets of the transcriptional coactivator Yap upregulated upon Nf2 deletion, suggesting that Nf2 may inhibit Yap activity. Consistent with this, protein levels and nuclear localization of Yap and its paralog Taz are increased in Nf2 mutants. Moreover, Yap deletion rescues the Nf2 mutant phenotype – demonstrating the functional importance of this regulation. These data uncover a key role for Nf2 and Yap/Taz in regulating NPC proliferation in the developing brain.
Mutation of neurofibromatosis 2 (NF2) results in nervous system tumours. Molecularly, Nf2 has diverse functions, regulating cell-cell junction formation and various signalling pathways, including the Hippo-Yap pathway. However, the roles of Nf2 in the nervous system, and how its loss promotes tumorigenesis, are poorly understood. Here (p. 3323), Xinwei Cao and co-workers analyse the consequences of Nf2 deletion in the dorsal telencephalon. Although the mutant mice are viable, they display significant brain malformations associated with neural progenitor cell (NPC) hyperproliferation. To determine how Nf2 limits NPC expansion, the authors performed a microarray analysis and found many known targets of the transcriptional coactivator Yap upregulated upon Nf2 deletion, suggesting that Nf2 may inhibit Yap activity. Consistent with this, protein levels and nuclear localization of Yap and its paralog Taz are increased in Nf2 mutants. Moreover, Yap deletion rescues the Nf2 mutant phenotype – demonstrating the functional importance of this regulation. These data uncover a key role for Nf2 and Yap/Taz in regulating NPC proliferation in the developing brain.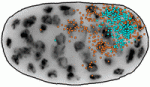 During embryogenesis, transcriptional regulation must be coordinated with growth and cell division, so that genes are turned on or off in the right cells at the right time. Arjun Raj and colleagues now investigate the coupling of gene expression and cell division in C. elegans (p. 3385). They find that global retardation of development by temperature change or gene mutation slows down the cell cycle, and this is accompanied by a similar delay in expression of particular developmental genes – so the synchrony between cell cycle and gene expression is retained. These findings suggest that transcription might be directly cell cycle dependent. However, mutations that cause cell cycle delays in specific lineages uncouple cell division and transcription, arguing against the onset of transcription being tied to a particular division cycle. Conversely, it is known that cell division in C. elegans embryos proceeds independently of zygotic transcription. Together, these data demonstrate that cell proliferation and gene expression are well synchronised, but raise the key question of how this synchrony is achieved.
During embryogenesis, transcriptional regulation must be coordinated with growth and cell division, so that genes are turned on or off in the right cells at the right time. Arjun Raj and colleagues now investigate the coupling of gene expression and cell division in C. elegans (p. 3385). They find that global retardation of development by temperature change or gene mutation slows down the cell cycle, and this is accompanied by a similar delay in expression of particular developmental genes – so the synchrony between cell cycle and gene expression is retained. These findings suggest that transcription might be directly cell cycle dependent. However, mutations that cause cell cycle delays in specific lineages uncouple cell division and transcription, arguing against the onset of transcription being tied to a particular division cycle. Conversely, it is known that cell division in C. elegans embryos proceeds independently of zygotic transcription. Together, these data demonstrate that cell proliferation and gene expression are well synchronised, but raise the key question of how this synchrony is achieved.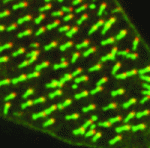 Cells lining the lumen of various organs, such as the lung airway and the female reproductive tract, are multiciliated, and all the cilia are oriented in the same direction to generate flow. But how is cilia orientation coordinated within cells and across tissues? Chris Kintner and colleagues use the epithelial cells of Xenopus embryos as a model to study multicilate cell differentiation. On p. 3468, they identify a new regulator of cilia polarisation, the coiled-coil protein bbof1. Bbof1 is expressed in multicilate cells and localises to the axoneme and the basal body – the structure that determines cilia orientation. Upon bbof1 depletion, motile cilia still form, but are unable to generate significant flow because their orientation is disturbed. Notably, bbof1 is not required for the initial phase of cilia polarisation, but rather for the later refinement step, and for stabilising the alignment. Although the mechanism by which bbof1 acts remains unclear, this work identifies a key factor regulating cilia orientation and function.
Cells lining the lumen of various organs, such as the lung airway and the female reproductive tract, are multiciliated, and all the cilia are oriented in the same direction to generate flow. But how is cilia orientation coordinated within cells and across tissues? Chris Kintner and colleagues use the epithelial cells of Xenopus embryos as a model to study multicilate cell differentiation. On p. 3468, they identify a new regulator of cilia polarisation, the coiled-coil protein bbof1. Bbof1 is expressed in multicilate cells and localises to the axoneme and the basal body – the structure that determines cilia orientation. Upon bbof1 depletion, motile cilia still form, but are unable to generate significant flow because their orientation is disturbed. Notably, bbof1 is not required for the initial phase of cilia polarisation, but rather for the later refinement step, and for stabilising the alignment. Although the mechanism by which bbof1 acts remains unclear, this work identifies a key factor regulating cilia orientation and function.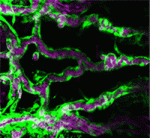 Blood flow through the developing vasculature regulates vessel formation – both via the distribution of endocrine factors, and via mechanical force-induced responses. Several signalling pathways are known to be involved in this process, including signalling via the TGFb receptor Alk1, whose activity promotes quiescence in newly formed arteries and whose expression is itself dependent upon blood flow. On p. 3403, Beth Roman and colleagues demonstrate that not only Alk1 expression but also its activity are dependent upon blood flow in developing zebrafish. They identify Bmp10 as the endogenous ligand for Alk1 in this context, and find that Bmp10 is exclusively expressed in the heart, and not in the vascular tissue. Through elegant experiments using embryos in which the heart has been stopped but alk1 expression restored, they show that Bmp10 injection can locally rescue Alk1 pathway activity and downstream transcriptional responses. Thus, their data suggest that blood flow is required to distribute cardiac-derived Bmp10 into the vasculature, where it activates Alk1 to promote quiescence in endothelial cells.
Blood flow through the developing vasculature regulates vessel formation – both via the distribution of endocrine factors, and via mechanical force-induced responses. Several signalling pathways are known to be involved in this process, including signalling via the TGFb receptor Alk1, whose activity promotes quiescence in newly formed arteries and whose expression is itself dependent upon blood flow. On p. 3403, Beth Roman and colleagues demonstrate that not only Alk1 expression but also its activity are dependent upon blood flow in developing zebrafish. They identify Bmp10 as the endogenous ligand for Alk1 in this context, and find that Bmp10 is exclusively expressed in the heart, and not in the vascular tissue. Through elegant experiments using embryos in which the heart has been stopped but alk1 expression restored, they show that Bmp10 injection can locally rescue Alk1 pathway activity and downstream transcriptional responses. Thus, their data suggest that blood flow is required to distribute cardiac-derived Bmp10 into the vasculature, where it activates Alk1 to promote quiescence in endothelial cells. Polycomb group proteins are chromatin regulators with highly conserved functions. The Polycomb repressive complex 2 (PRC2) methylates H3K27 to stably silence target genes, including the HOX genes in Drosophila. More recently, Utx and Jmjd3 demethylases were found to reverse PRC2-mediated H3K27 methylation, and it has been suggested that a dynamic cycle of methylation and demethylation is required for appropriate regulation of gene expression. Now, Ömer Copur and Jürg Müller challenge this view (p. 3478), via the analysis of Drosophila Utx mutants. Lack of zygotic Utx function has no effect on Drosophila development, although mutant adults die shortly after hatching. Loss of both maternal and zygotic Utx, however, leads to larval death and to defects in HOX gene expression – in both the embryo and larval imaginal discs. Thus, it appears that Utx in Drosophila – and, by inference, H3K27 demethylation – is required only at early stages to set up the patterns of HOX expression; it is largely dispensable later in development, suggesting that H3K27 methylation may in fact be very stable.
Polycomb group proteins are chromatin regulators with highly conserved functions. The Polycomb repressive complex 2 (PRC2) methylates H3K27 to stably silence target genes, including the HOX genes in Drosophila. More recently, Utx and Jmjd3 demethylases were found to reverse PRC2-mediated H3K27 methylation, and it has been suggested that a dynamic cycle of methylation and demethylation is required for appropriate regulation of gene expression. Now, Ömer Copur and Jürg Müller challenge this view (p. 3478), via the analysis of Drosophila Utx mutants. Lack of zygotic Utx function has no effect on Drosophila development, although mutant adults die shortly after hatching. Loss of both maternal and zygotic Utx, however, leads to larval death and to defects in HOX gene expression – in both the embryo and larval imaginal discs. Thus, it appears that Utx in Drosophila – and, by inference, H3K27 demethylation – is required only at early stages to set up the patterns of HOX expression; it is largely dispensable later in development, suggesting that H3K27 methylation may in fact be very stable. Integrins mediate cell-matrix adhesion and are also capable of inducing intracellular signalling cascades to regulate cell proliferation, differentiation and other cell behaviours. In vitro, disruption of β1 integrin function has been shown to affect various aspects of pancreatic β-cell activity. On p. 3360, Vincenzo Cirulli and co-workers analyse the consequences of deleting β1 integrin in β-cells in vivo in mice. The mutant mice have smaller pancreatic islets that exhibit matrix adhesion defects when cultured in vitro. Notably, cell proliferation is severely impaired in the mutant β-cells, and the expression of cell cycle regulators is highly abnormal. However, these cells are able to differentiate properly and to express insulin, and are glucose responsive; in fact, they show increased levels of insulin and the mutant mice show no signs of diabetes. These results highlight differences between the ascribed functions of β1-integrin in vitro versus in vivo and define its key role in promoting proliferation during pancreatic islet development.
Integrins mediate cell-matrix adhesion and are also capable of inducing intracellular signalling cascades to regulate cell proliferation, differentiation and other cell behaviours. In vitro, disruption of β1 integrin function has been shown to affect various aspects of pancreatic β-cell activity. On p. 3360, Vincenzo Cirulli and co-workers analyse the consequences of deleting β1 integrin in β-cells in vivo in mice. The mutant mice have smaller pancreatic islets that exhibit matrix adhesion defects when cultured in vitro. Notably, cell proliferation is severely impaired in the mutant β-cells, and the expression of cell cycle regulators is highly abnormal. However, these cells are able to differentiate properly and to express insulin, and are glucose responsive; in fact, they show increased levels of insulin and the mutant mice show no signs of diabetes. These results highlight differences between the ascribed functions of β1-integrin in vitro versus in vivo and define its key role in promoting proliferation during pancreatic islet development.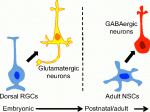 Eduardo Sequerro and colleagues review studies of postnatal and adult neurogenesis, challenging the notion that fixed genetic programs restrict neuronal fate. They hypothesize that the adult brain maintains plastic neural stem cells that are capable of responding to changes in environmental cues and generating diverse neuronal types. See the Hypothesis article on p. 3303
Eduardo Sequerro and colleagues review studies of postnatal and adult neurogenesis, challenging the notion that fixed genetic programs restrict neuronal fate. They hypothesize that the adult brain maintains plastic neural stem cells that are capable of responding to changes in environmental cues and generating diverse neuronal types. See the Hypothesis article on p. 3303 Weisheng Chen and Tom Maniatis provide a concise overview of the molecular and cellular biology of clustered Pcdhs, highlighting how they generate single cell diversity in the vertebrate nervous system and how such diversity may be used in neural circuit assembly. See the Development at a Glance poster article on p. 3297
Weisheng Chen and Tom Maniatis provide a concise overview of the molecular and cellular biology of clustered Pcdhs, highlighting how they generate single cell diversity in the vertebrate nervous system and how such diversity may be used in neural circuit assembly. See the Development at a Glance poster article on p. 3297

 (No Ratings Yet)
(No Ratings Yet)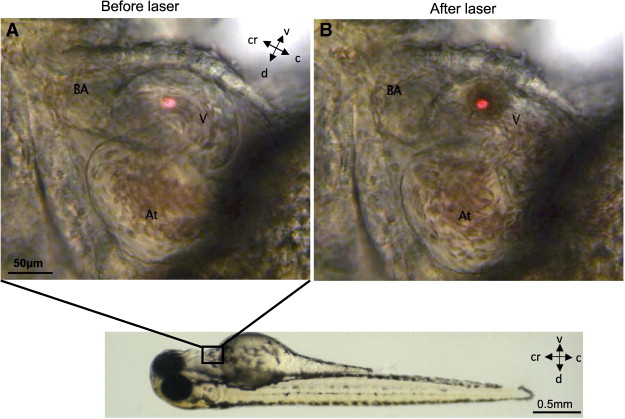
 (1 votes)
(1 votes) (13 votes)
(13 votes)




