In Development this week (Vol. 140, Issue 17)
Posted by Seema Grewal, on 13 August 2013
Here are the highlights from the current issue of Development:
GDNF’s got a nerve
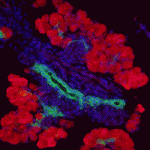 Innervation of the mammalian pancreas is crucial for endocrine and exocrine function. Neural crest cells that give rise to neural progenitor cells are responsible for intrinsic pancreatic innervation, but the specific cues that guide this process in the developing embryo are largely unknown. Now, on page 3669, David Cano and colleagues identify glial cell line-derived neurotrophic factor (GDNF) as a key player in this process. They show that GDNF is expressed in the developing pancreatic epithelium and that conditional GDNF inactivation results in a marked reduction of neuronal and glial cells in both newborn and juvenile mice. In juveniles, parasympathetic innervation density decreased by just over half and appeared to be selectively reduced in pancreatic islets. Using wild-type E11.5 pancreatic explants, the authors demonstrate that GDNF can induce neural progenitor expansion and functions to promote the migration and differentiation of these progenitors. These results present a previously unappreciated role for GDNF in the regulation of neural crest-derived neural progenitor cells and the subsequent intrinsic innervation of the developing pancreas.
Innervation of the mammalian pancreas is crucial for endocrine and exocrine function. Neural crest cells that give rise to neural progenitor cells are responsible for intrinsic pancreatic innervation, but the specific cues that guide this process in the developing embryo are largely unknown. Now, on page 3669, David Cano and colleagues identify glial cell line-derived neurotrophic factor (GDNF) as a key player in this process. They show that GDNF is expressed in the developing pancreatic epithelium and that conditional GDNF inactivation results in a marked reduction of neuronal and glial cells in both newborn and juvenile mice. In juveniles, parasympathetic innervation density decreased by just over half and appeared to be selectively reduced in pancreatic islets. Using wild-type E11.5 pancreatic explants, the authors demonstrate that GDNF can induce neural progenitor expansion and functions to promote the migration and differentiation of these progenitors. These results present a previously unappreciated role for GDNF in the regulation of neural crest-derived neural progenitor cells and the subsequent intrinsic innervation of the developing pancreas.
Ser2-ious changes in the germline-soma
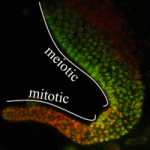 Transcriptional elongation via RNA polymerase II (Pol II) is an essential component of gene expression. It has long been thought that a necessary step of elongation is the serine 2 phosphorylation (Ser2-P) of Pol II, which is catalysed by a CDK-9/cyclin T complex called P-TEFb. Although this dogma holds true in the soma, Elizabeth Bowman, William Kelly and colleagues now present evidence (see p. 3703) that Ser2-P of Pol II in the C. elegans germline occurs via a P-TEFb-independent mechanism primarily involving CDK-12, not CDK-9, activity. Nonetheless, CDK-9 is required for C. elegans germline development, raising the possibility of an alternative essential function for CDK-9. Conversely, loss of CDK-12 and subsequent Ser2-P had little effect on germline development, suggesting a possible alternative mechanism for regulation of Pol II transcriptional elongation in the germline. These data demonstrate that Ser2-P can occur independently of P-TEFb in at least one context, and further suggest that alternative mechanisms of Pol II elongation activity might be important in establishing and/or maintaining the germline-soma distinction.
Transcriptional elongation via RNA polymerase II (Pol II) is an essential component of gene expression. It has long been thought that a necessary step of elongation is the serine 2 phosphorylation (Ser2-P) of Pol II, which is catalysed by a CDK-9/cyclin T complex called P-TEFb. Although this dogma holds true in the soma, Elizabeth Bowman, William Kelly and colleagues now present evidence (see p. 3703) that Ser2-P of Pol II in the C. elegans germline occurs via a P-TEFb-independent mechanism primarily involving CDK-12, not CDK-9, activity. Nonetheless, CDK-9 is required for C. elegans germline development, raising the possibility of an alternative essential function for CDK-9. Conversely, loss of CDK-12 and subsequent Ser2-P had little effect on germline development, suggesting a possible alternative mechanism for regulation of Pol II transcriptional elongation in the germline. These data demonstrate that Ser2-P can occur independently of P-TEFb in at least one context, and further suggest that alternative mechanisms of Pol II elongation activity might be important in establishing and/or maintaining the germline-soma distinction.
In good sTEAD4 energy homeostasis
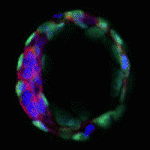 The blastocoel of mammalian embryos is a fluid-filled cavity surrounded by a monolayer of trophectoderm, and its formation marks one of the earliest stages of embryonic development. During this process, the trophectoderm undergoes a metabolic shift toward oxidative phosphorylation, which increases the level of reactive oxygen species (ROS) and places the blastocyst under oxidative stress. In this issue (p. 3680), Melvin DePamphilis and Kotaro Kaneko uncover a role for TEAD4, which was previously thought to specify trophectoderm, in managing oxidative stress and establishing energy homeostasis during murine blastocoel formation. The authors show that under conditions that mimic the in vivo environment, TEAD4 is necessary for trophectoderm and blastocoel formation, but that this requirement is lost when conditions are manipulated to minimise oxidative stress. The authors demonstrate that Tead4-/- mitochondria have a reduced membrane potential in trophoblast giant cells, and furthermore that TEAD4 could localise to mitochondria, possibly to prevent accumulation of ROS. These data reveal a metabolic requirement for TEAD4 in the developing mouse embryo, rather than in trophectoderm lineage specification.
The blastocoel of mammalian embryos is a fluid-filled cavity surrounded by a monolayer of trophectoderm, and its formation marks one of the earliest stages of embryonic development. During this process, the trophectoderm undergoes a metabolic shift toward oxidative phosphorylation, which increases the level of reactive oxygen species (ROS) and places the blastocyst under oxidative stress. In this issue (p. 3680), Melvin DePamphilis and Kotaro Kaneko uncover a role for TEAD4, which was previously thought to specify trophectoderm, in managing oxidative stress and establishing energy homeostasis during murine blastocoel formation. The authors show that under conditions that mimic the in vivo environment, TEAD4 is necessary for trophectoderm and blastocoel formation, but that this requirement is lost when conditions are manipulated to minimise oxidative stress. The authors demonstrate that Tead4-/- mitochondria have a reduced membrane potential in trophoblast giant cells, and furthermore that TEAD4 could localise to mitochondria, possibly to prevent accumulation of ROS. These data reveal a metabolic requirement for TEAD4 in the developing mouse embryo, rather than in trophectoderm lineage specification.
Regeneration reoriented
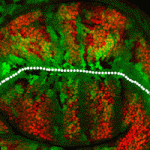 The Drosophila wing primordium is a simple epithelial structure with significant regenerative capacity. As in any regenerative system, it is essential to maintain correct tissue size and shape during the repair process, yet little is known about the mechanisms that enable such control. In this issue (p. 3541), Florenci Serras and colleagues demonstrate that regeneration of the Drosophila wing involves respecification of cell fate as well as reorientation of cell division in order to drive intercalary growth. Following genetic ablation of wing cells, the authors observed a respecification of vein and intervein fates, which was independent of Hedgehog and Decapentaplegic morphogen activity. The authors identified the proteins Fat (Ft) and Crumbs (Crb) as required for the reorientation of cell division, and showed that mutations in ft or crb lead to misorientation of the mitotic spindle as well as to Yorkie-driven excessive proliferation, resulting in malformed wings. This elegant Drosophila model provides novel insight into the mechanisms of epithelial regeneration and wound repair.
The Drosophila wing primordium is a simple epithelial structure with significant regenerative capacity. As in any regenerative system, it is essential to maintain correct tissue size and shape during the repair process, yet little is known about the mechanisms that enable such control. In this issue (p. 3541), Florenci Serras and colleagues demonstrate that regeneration of the Drosophila wing involves respecification of cell fate as well as reorientation of cell division in order to drive intercalary growth. Following genetic ablation of wing cells, the authors observed a respecification of vein and intervein fates, which was independent of Hedgehog and Decapentaplegic morphogen activity. The authors identified the proteins Fat (Ft) and Crumbs (Crb) as required for the reorientation of cell division, and showed that mutations in ft or crb lead to misorientation of the mitotic spindle as well as to Yorkie-driven excessive proliferation, resulting in malformed wings. This elegant Drosophila model provides novel insight into the mechanisms of epithelial regeneration and wound repair.
Hoi Polloi muscles in on myogenesis
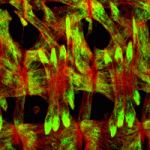 The proper formation of somatic muscle depends on morphological and molecular events that orchestrate the specification, maturation and terminal differentiation of muscle precursor cells during development. Many of the transcriptional regulatory networks that regulate these processes have been defined, but little is known about the possible post-transcriptional mechanisms that might also be involved. In this issue (p. 3645), Eric Olson, Aaron Johnson and colleagues conduct a genetic screen for regulators of muscle morphology in Drosophila and identify Hoi Polloi (Hoip), a putative RNA-binding protein with two distinct roles in myogenesis. First, the authors show that Hoip is required for myotube elongation, since elongation failed to initiate in hoip mutants. Second, they show that Hoip is necessary for the expression of sarcomeric proteins Myosin heavy chain (MHC) and Tropomyosin. Importantly, MHC protein expression was restored in the hoip mutant via delivery of a prespliced MHC transcript, suggesting a role for Hoip in pre-mRNA splicing. These data demonstrate a novel and tissue-specific, post-transcriptional role for Hoip in Drosophila development.
The proper formation of somatic muscle depends on morphological and molecular events that orchestrate the specification, maturation and terminal differentiation of muscle precursor cells during development. Many of the transcriptional regulatory networks that regulate these processes have been defined, but little is known about the possible post-transcriptional mechanisms that might also be involved. In this issue (p. 3645), Eric Olson, Aaron Johnson and colleagues conduct a genetic screen for regulators of muscle morphology in Drosophila and identify Hoi Polloi (Hoip), a putative RNA-binding protein with two distinct roles in myogenesis. First, the authors show that Hoip is required for myotube elongation, since elongation failed to initiate in hoip mutants. Second, they show that Hoip is necessary for the expression of sarcomeric proteins Myosin heavy chain (MHC) and Tropomyosin. Importantly, MHC protein expression was restored in the hoip mutant via delivery of a prespliced MHC transcript, suggesting a role for Hoip in pre-mRNA splicing. These data demonstrate a novel and tissue-specific, post-transcriptional role for Hoip in Drosophila development.
Ubiquitin depletion stirs up sterility
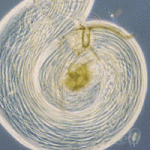 The ubiquitin proteasome system regulates protein expression at the post-translational level by tagging certain proteins with ubiquitin and thereby marking them for degradation by the proteasome complex. This system is highly conserved and is used by almost every eukaryotic cell to degrade and recycle proteins. In this issue (p. 3522), Margaret Fuller and colleagues uncover a surprisingly specific role for the polyubiquitin Ubi-p63E, encoded by magellan (magn), in the Drosophila spermatogenetic programme. Loss-of-function magn mutants fail to maintain the free ubiquitin pool specifically in the testes, which ultimately results in meiotic arrest and sterility. The authors show that Ubi-p63E is required for normal meiotic chromatin condensation, cell cycle progression and spermatid differentiation, whereas the expression of spermatocyte genes was largely unaffected in the magn mutant. The observed phenotype is likely to be specific to the magn mutant, as knockdown of proteasome subunits had more widespread and severe effects. These data uncover a novel and highly specific mechanism of post-translational regulation via ubiquitin homeostasis in the developing male germline.
The ubiquitin proteasome system regulates protein expression at the post-translational level by tagging certain proteins with ubiquitin and thereby marking them for degradation by the proteasome complex. This system is highly conserved and is used by almost every eukaryotic cell to degrade and recycle proteins. In this issue (p. 3522), Margaret Fuller and colleagues uncover a surprisingly specific role for the polyubiquitin Ubi-p63E, encoded by magellan (magn), in the Drosophila spermatogenetic programme. Loss-of-function magn mutants fail to maintain the free ubiquitin pool specifically in the testes, which ultimately results in meiotic arrest and sterility. The authors show that Ubi-p63E is required for normal meiotic chromatin condensation, cell cycle progression and spermatid differentiation, whereas the expression of spermatocyte genes was largely unaffected in the magn mutant. The observed phenotype is likely to be specific to the magn mutant, as knockdown of proteasome subunits had more widespread and severe effects. These data uncover a novel and highly specific mechanism of post-translational regulation via ubiquitin homeostasis in the developing male germline.
PLUS…
A molecular basis for developmental plasticity in early mammalian embryos
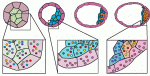 Early mammalian embryos exhibit remarkable plasticity, and recent studies of mouse embryos implicate a network of transcription factors in governing this plasticity and the establishment of embryonic lineages. Here, Alfonso Martinez Arias and colleagues summarise this information, link it to classical embryology and propose a molecular framework for the establishment and regulation of developmental plasticity. See the Hypothesis article on p. 3499
Early mammalian embryos exhibit remarkable plasticity, and recent studies of mouse embryos implicate a network of transcription factors in governing this plasticity and the establishment of embryonic lineages. Here, Alfonso Martinez Arias and colleagues summarise this information, link it to classical embryology and propose a molecular framework for the establishment and regulation of developmental plasticity. See the Hypothesis article on p. 3499
An interview with François Guillemot
 François Guillemot heads the Division of Molecular Neurobiology at the MRC National Institute of Medical Research in London, where his research focuses on the transcriptional control of neurogenesis and the epigenetic regulation of gene expression in neural development. François recently became an editor for Development, and we asked him about his research and career, as well as his lab’s future move to the Crick Institute. See the Spotlight article on p. 3497
François Guillemot heads the Division of Molecular Neurobiology at the MRC National Institute of Medical Research in London, where his research focuses on the transcriptional control of neurogenesis and the epigenetic regulation of gene expression in neural development. François recently became an editor for Development, and we asked him about his research and career, as well as his lab’s future move to the Crick Institute. See the Spotlight article on p. 3497


 (No Ratings Yet)
(No Ratings Yet)
 (5 votes)
(5 votes)