The people behind the papers – Emily Lo and Keiko Torii
Posted by the Node Interviews, on 3 January 2021
This interview, the 87th in our series, was published in Development last year.
The patterning of stomata – the pores in the plant epidermis that facilitate gas exchange and water control – is regulated by a family of small secreted peptides. A new paper in Development analyses the effective ranges of two such peptides, borrowing a statistical technique used by astrophysicists to investigate the distribution and patterning of galaxies. We caught up with authors Emily Lo, who worked on the project when an undergraduate at the University of Washington (UW), and her supervisor Keiko Torii, who recently moved her lab from UW to The University of Texas at Austin (where she is Professor and Johnson & Johnson Centennial Chair in Plant Cell Biology), to hear more about the story.

Keiko, can you give us your scientific biography and the questions your lab is trying to answer?
KT: As a plant developmental biologist, I was always super fascinated by how dazzling arrays of functional, beautiful patterns emerge during development and how the external environment, where plants thrive, shapes the manifestation of such functional patterns. My main focus is to tackle such observations through understanding the molecular and genetic basis of cell-cell communication, ‘how plant cells talk to each other to generate functional patterns’.
I received my PhD and did my first short postdoc in Japan, where I identified the causal gene for the Arabidopsis mutant, erecta, which exhibits short stature and altered organ shape. It turned out that the ERECTA gene encodes a putative receptor kinase (collectively known as ‘Receptor-like Kinase’ or ‘RLK’), and it was the first report that this type of putative peptide receptor regulates plant growth and development. Later, after I obtained a tenure-track Assistant Professor position at the UW in Seattle, our research elucidated that ERECTA-family RLKs perceive a family of peptides to enforce proper stomatal patterning, which is the basis of this work.
In the Fall of 2019, I accepted the Johnson & Johnson Centennial Chair in Plant Cell Biology at the Department of Molecular Biosciences, The University of Texas at Austin. Currently I am also a Howard Hughes Medical Investigator.
And Emily – how did you come to work in Keiko’s lab on this project?
EL: I was lucky enough to be hired as an undergrad in Keiko’s lab during my freshman year at UW, and I stayed on until I graduated. It’s a rare opportunity to have such a long research experience as an undergrad, so I was able to devote a substantial amount of time to this project. Keiko introduced me to this fascinating project using mosaic fluorescent sectors to track peptide expression, which had been initiated by Dr. Takeshi Kuroha, a previous postdoctoral fellow, and Janelle Sagawa, a previous undergrad researcher (both acknowledged in the manuscript). So right away I could start growing seedlings, generating these mosaic sectors, and imaging on the confocal microscope. It was great fun! I graduated before we were able to complete the project, so Scott [Zeng] developed the majority of the SPACE pipeline after I left the lab.
How has your research been affected by the COVID-19 pandemic?
KT: Unfortunately, my lab and research program got hit really hard by the COVID-19 situation, because we had just relocated from Seattle WA to Austin TX right before the pandemic hit. Our brand-new lab at UT Austin was finally operating in the beginning of 2020 when we had to suddenly shut it down. Like myself, almost all the new lab members were new to Texas (or the Southern part of USA), and many were separated from family. So, staying at home in an unfamiliar city was stressful to everyone, and I truly thank my lab members for being positive and hanging together during this exceptionally difficult time. As for this manuscript, since we were at the phase of drafting a manuscript (lucky us!), Scott and I completed the manuscript during the full shut down phase, with thoughtful inputs from Emily and other co-authors. New online technology, such as Zoom, helped us work together, remotely.
EL: For this project, it was fortunate in that all experiments were completed and we were at the data analysis phase before COVID hit, so we were able to complete it through online communication (and because I’m located in Baltimore, I’d been communicating solely online anyway). For my current research at Hopkins, we were in an Essential-Only phase from March to June, in which no new experiments were allowed to begin; luckily, at that point I did have some computational analysis to catch up on. In mid-June we had our Phase-I reopening, so I’ve been able to resume many of my experiments, though of course progress is still limited by how much time we’re allotted in the lab.
Why have the signalling ranges of peptides like EPF1 and Stomagen been hard to assess, prior to your paper?
EL & KT: Whether secreted peptides or small chemical hormones, how far the signal moves is a fundamental question of pattern formation in development. But precisely quantifying the distance is not trivial. For direct observation, one could tag fluorescent proteins (or exogenously synthesize a peptide conjugated with a fluorophore). In a strict sense, however, such modifications change the size and property of peptides or chemical signals. Alternatively, one could develop sensors (such as a FRET sensor) that detect the existence of peptides or chemical signals.
We have previously shown that EPIDERMAL PATTERNING FACTOR (EPF) family members of secreted peptides fine-tune patterning of stomata on the plant epidermis by competitive binding to the same receptor. Because of the flat, two-dimensional nature of the developing leaf epidermis, we thought that our system would be a great model for understanding how far the secreted peptides influence tissue patterning, rather than directly observing its movement.
Can you give us the key results of the paper in a paragraph?
EL & KT: Using recombination-based mosaic sectors to overexpress signaling peptides EPF1 and Stomagen, which inhibit and promote stomatal development, respectively, we determined the effective ranges of these peptides in Arabidopsis cotyledons. We developed a quantitative pipeline to model stomatal distributions across the cotyledon in response to peptide overexpression, which we named SPACE (stomata patterning autocorrelation on epidermis), an homage to the astrophysics origin of the autocorrelation approach. We found that the inhibitor peptide EPF1 has a longer effective range than the activator peptide STOMAGEN, and that the patterning effects of peptide overexpression are limited to a local range rather than the global cotyledon.
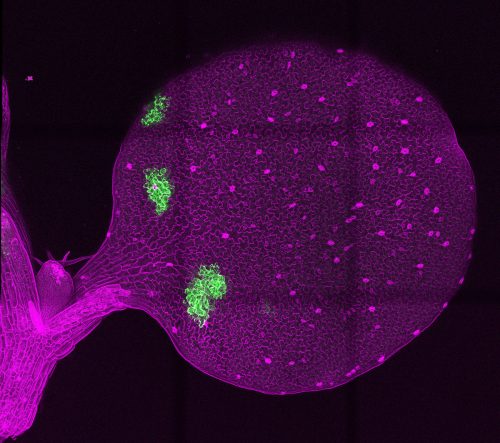
How did you come upon the idea of borrowing an astrophysical technique to look at stomatal patterning?
KT: At the initial stage of our research, we were able to produce chimeras via heat-shock Cre/lox recombination, but we could not think of how to actually ‘calculate’ the effective distance of the peptides. Initially, we tried to set bin range (such as 100 μm, 200 μm, etc.) from each sector border. However, because of the complex geometry of pavement cells as well as the unique size, shape and location of individual sectors, we could not figure out how to normalize stomatal distribution surrounding each sector.
One morning in the kitchen, I was talking about this problem to my spouse, who is a theoretical physicist studying String Theory. When I was drawing a cartoon of a simple leaf (essentially an oval) with lots of stomata (essentially dots inside the oval), he told me, ‘this sounds familiar to me. I think my colleague, Miguel, is addressing the exact same problem – except that in his case, it’s the distribution of galaxies in the Universe’. I immediately e-mailed Prof. Miguel Morales of UW Physics. I thought that he might think I was crazy, but to my pleasant surprise, Miguel and his postdoc Dr Bryna Hazelton were very excited to hear about our research and the potential of the spatial autocorrelation statistics that they utilize for astrophysics in solving questions in plant development. Bryna mentored Emily in programming for the spatial autocorrelation analysis.
My Physics colleagues generously gave me the opportunity to give a 15 min talk to incoming Physics Graduate Students. I discussed the principles of spatial patterning in biological systems and introduced Alan Turing’s reaction-diffusion model; luckily, Scott remembered my brief talk and was curious enough to join my lab to tackle this problem after Emily’s graduation.
EL: For me it started when Keiko suggested we meet with Miguel and Bryna. When we explained that we were trying to generate a metric of epidermal spatial patterning, they almost immediately suggested looking into autocorrelation, an astrophysics method for analyzing galaxy distributions/patterning. Bryna works at the UW eScience Institute, and has a goal to empower the next generation of researchers and students to answer fundamental questions in complex or noisy data. Working with her to develop code to analyze our epidermal patterning questions was a really wonderful and fruitful experience, and that was the starting point for our collaboration and the development of the SPACE pipeline.
Why do you think the stomatal inhibitor EPF1 is able to travel farther than the activator Stomagen?
EL & KT: To clarify your question, the goal of our study was to determine how far these peptides act or have an effect, not necessarily the physical distance they travel. EPF1 has a longer range in terms of developmental outcome than Stomagen, but not necessarily diffusion distance. The question of their differing ranges is complex because stomatal lineage cells (which are induced by Stomagen) will themselves secrete EPF1, acting as a negative-feedback loop; in other words, the peptides are not acting in isolation. Our quantitative determination of peptide effective range is fascinating because in the Turing theory of pattern formation, a short-range activator often interacts with a longer-range inhibitor to achieve a self-regulating, periodic pattern. The relationship between Stomagen and EPF1 might be one such example of this effect to achieve regular, ordered stomatal spacing across the epidermis.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
EL: After we’d established that the mosaic sector generation system indeed worked, we wanted to observe the potential global effects of peptide overexpression (from within the restricted mosaic sectors) across the entire cotyledon, so I switched to a confocal microscope that had tile-scanning functionality. The stitched image I got of the entire cotyledon surface was very beautiful; getting that first image was a hugely satisfying and validating moment for me.
Getting that first image was a hugely satisfying and validating moment for me
And what about the flipside: any moments of frustration or despair?
EL: The initial months were the most difficult experimentally for me. There were a lot of experimental skills that required finesse, for example sowing tiny Arabidopsis seeds individually on plates or mounting the cotyledons on glass slides completely flat without folding or tearing, etc. The way I overcame these challenges was practice and repetition: those are just skills that are gained slowly over time.
I understand you’ve now left Keiko’s lab – what are you doing now?
EL: I’m currently pursuing my PhD in Biomedical Engineering at Johns Hopkins, in the labs of Prof. Patrick Cahan and Prof. Andrew Feinberg. I study how changes in cell identity relate to cancer initiation in the context of pancreatic ductal adenocarcinoma: one of the most lethal malignancies in the US.
Even though I work on human disease research now, plant research is still very important to me, especially given the adaptations humans will have to make to agricultural practices in response to global climate change. Particularly, I think optimizing crop water consumption will be a key strategy in the next several decades to reach necessary agricultural yields in fluctuating environmental conditions.
Where will this story take the Torii lab?
KT: Stomatal patterning and distribution is critical for plant productivity and water use efficiency, and many different genetic and environmental factors (such as temperature, light, CO2 and drought) influence stomatal number, density and distribution. There is a long history in Plant Physiology of studying these traits. Yet, essentially, there are two ways to quantify these traits: stomata density (number of stomata per given area) and stomatal index (number of stomata per total epidermal cells). Different genetic backgrounds and environmental conditions may influence number, density or distribution in unique ways, but often such information is lost by simply presenting bar graphs of stomatal density and index. So, the natural next direction is to apply SPACE pipelines to describe different patterns of stomata, in different ages of Arabidopsis leaves or under different genetic/environmental conditions. Further harnessing the SPACE pipeline to quantitatively characterize stomatal patterning of agronomically important plant species, such as tomato and cereals, may reveal some important characteristics. Finally, we are looking for developmental biologists studying pattern formation of any systems who are brave enough to try out our SPACE pipeline to quantitatively characterize their systems.
Finally, let’s move outside the lab – what do you like to do in your spare time in Baltimore and Austin?
KT: Well…since I just relocated alone to a small one-bedroom apartment in Austin, and then the COVID-19 stay-at-home order was put in place, I really did not have much time to explore my new city. My spouse and children were supposed to move to Austin from Seattle this summer, but this got postponed due to COVID-19. I really hope that, when we (the USA) manage to get the pandemic under control, I can explore the Texas Hill Country and enjoy its natural beauty, and see bluebonnet wildflowers blooming for the first time in my life!
EL: I’m an avid baker, cook and gardener. During COVID-19, I’ve boarded the sourdough starter bandwagon as an at-home quarantine activity. And as much as possible, I’ve tried to stay in touch with friends and family across the country and the world by video chatting.


 (No Ratings Yet)
(No Ratings Yet)