The people behind the papers – Jinjin Zhu & Justin Kumar
Posted by the Node Interviews, on 9 April 2018
Cell fate commitment relies on both activation of appropriate genes and suppression of inappropriate ones. Polycomb group proteins are known to be crucial epigenetic silencers of developmental genes, but the manner by which they control fate in vivo, and the relative roles of different Polycomb proteins in silencing, have remained unclear. A new paper in Development tackles this problem using the Drosophila eye a developmental model – we caught up with authors Jinjin Zhu and Justin Kumar, Professor of Biology at Indiana University in Bloomington, to find out more.

Justin, can you give us your scientific biography and the questions your lab is trying to answer?
JK I started my career in Drosophila eye development while I was an undergraduate in the laboratory of Karl Fryxell at the University of California, Riverside. He was a wonderful mentor and it was my time in his lab that convinced me that I wanted to be a professor one day. While I was in Karl’s lab, I read Don Ready’s seminal paper on the morphogenetic furrow (Ready et al., 1976) and was totally amazed by the cellular mechanism of pattern formation. From the images in the paper, I could see a field of undifferentiated cells being transformed into the periodic units of photoreceptor clusters right before my eyes. I knew then that I wanted to join his lab for my PhD studies. Being in Don’s lab at Purdue University as a graduate student was a privilege. He taught me how to love the fly eye for its own sake and to appreciate its intrinsic beauty.
I then went on to do my post-doctoral fellowship with Kevin Moses initially at the University of Southern California and then at Emory University. It was in Kevin’s lab that I finally settled on the research questions that still drive me today. I stumbled on what I thought to be an astonishing phenotype. Quite by accident I discovered that manipulations of the Notch and EGF Receptor signalling pathways led to the homeotic transformation of the eye into an antenna. While Hox mutants change entire body segments, I was able to observe fate transformations occurring within a single imaginal disc. When I joined the faculty of Indiana University I set out to understand how the fly eye-antennal disc is first set apart from the other discs (i.e. leg, wing, haltere, genital) and then how it is later subdivided into distinct territories such as the compound eye, ocelli, antenna, maxillary palps, and head epidermis. Over the years my research group has discovered that while gene regulatory networks promote fate specification, growth, and patterning, they also influence development by repressing alternate and inappropriate tissue fates. My current interests are to understand how the retinal determination gene regulatory network cooperates with signalling pathways and epigenetic complexes to prevent the eye from adopting distant fates such as the wing and more local fates such as the head epidermis.
And Jinjin how did you come to be involved with this project?
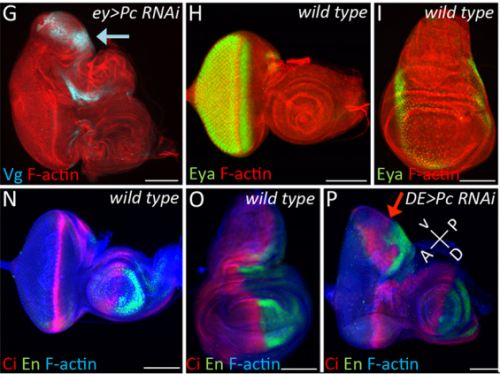
JZ I was really interested in the fate transformation caused by mis-expression of Hox genes when I was in college. When I joined the Justin’s lab, I did a genetic screen to find upstream regulators of eyeless in the developing eye disc. I knocked down eyeless and Sfmbt together and found this amazing eye-to-wing transformation phenotype. Meanwhile, Ali Ordway (second author) joined the lab and decided to screen other PcG proteins. She knocked down Pc and saw similar phenotypes. We were both fascinated by the images of these chimera tissue, in which the dorsal part of the eye disc turned into a wing disc while the ventral part remained as an eye disc, so we decided to figure out what happened to these mutant discs.
How did you come to be interested in the phenomenon of transdetermination?
JK I got interested in transdetermination purely by accident. When I joined Don’s lab in 1991, I made sure that I read every paper that he had published. In one of his papers, he and Ricky Lebovitz had transplanted eye-antennal disc fragments into host larvae and then recovered the tissue after the host had emerged as an adult (Lebovitz and Ready, 1986). These experiments were aimed at understanding if the morphogenetic furrow was pushed or pulled across the eye field. I was really intrigued by the disc transplantation method used in his paper so I started reading about the history of this method, which I learned was pioneered by Beadle and Ephrussi in the 1930s. In the course of these readings I came across the work of Ernst Hadorn. It was from his papers that I learned about the concepts of determination and transdetermination. When Jinjin and I saw the eye to wing transformation, it reminded me of the fact that Hadorn remarked that the eye could only transdetermine into a wing. And it was at that point that I realized that the loss of PcG and Pax6 that we noticed gave the eye to wing switch might be the molecular explanation for the eye to wing transdetermination event that Hadorn observed many decades ago.
Can you give us the key results of the paper in a paragraph?
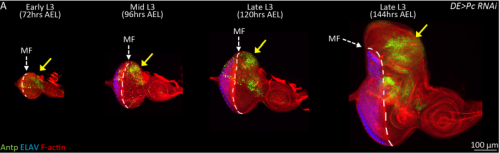
JK In this paper we demonstrate that the Pax6 transcription factor and the Polycomb group (PcG) of epigenetic silencers prevent the eye from adopting a wing fate. This decision is made early in development during the last stages of embryogenesis and during the first larval instar. The eye transforms into a wing because the chromatin around the Antp locus fails to be compacted thereby allowing for its activation by the zinc finger transcription factor Teashirt (Tsh), which is normally expressed in and required for the specification of the eye. Our findings suggest that in addition to promoting the primary fate of a tissue or organ, gene regulatory networks must play defense and suppress the activation of inappropriate selector genes and/or entire gene regulatory networks. This repressive activity appears to require cooperation from epigenetic silencing complexes such as PcG.
Why do you think the dorsal region of the eye might be more susceptible to wing transformation than the ventral region?
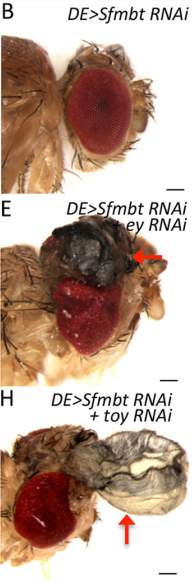
JZ This is a very interesting question and I think the answer lies down to the gene regulatory network controlling dorsal eye disc. 1) Wingless (wg), which is normaly required for wing development, is expressed in a higher level in the dorsal eye disc than in its ventral compartment. 2) engrailed (en) and cubitus interruptus (ci), which regulates A/P compartment of the wing disc also meets in the dorsal portion of the eye disc. Thus, the expression pattern of the endogenous genes in the dorsal eye disc contributes a lot to the eye-to-wing fate transformation. In addition, the dorsal eye disc is more susceptible to adopt wing fate because the level of Pax6 is significantly lower in the dorsal eye disc, especially in the region which will become the future dorsal head capsule. We have demonstrated that eye disc is more resistant to loss of Sfmbt function than the antennal disc. This is very likely due to the presence of Pax6 in the eye progenitor cells, because simultaneously knocking down Pax6 and Sfmbt induces the fate transformation.
It must have been particularly satisfying to discover the molecular underpinnings of phenomena first described in the pre-molecular biology age?
JK Yes, it was indeed satisfying to provide a modern perspective to an old problem/observation. In general I really enjoy reading the literature from the pre-molecular age – that is how I got interested in transdetermination in the first place. I also like scour the older literature for problems in eye development that were once studied but later abandoned due to the lack of the right genetic or molecular tools. If you look carefully enough, there is a wealth of such studies out there that are ripe for a modern perspective. For example, one of the exciting new areas of research in my laboratory today is the role that the peripodial epithelium, a tissue that overlies the eye-antennal disc, plays in development. There were several papers in the 1970s that suggested that its role was limited to the fusion of the two eye-antennal discs during pupal development. However, between 2000-2002 several laboratories provided evidence that signalling existed between these two tissues. But very little has been done since on this topic. Now several researchers in my lab are looking at the role that transcriptional networks in the peripodial epithelium play in promoting the fates of the eye-antennal disc.
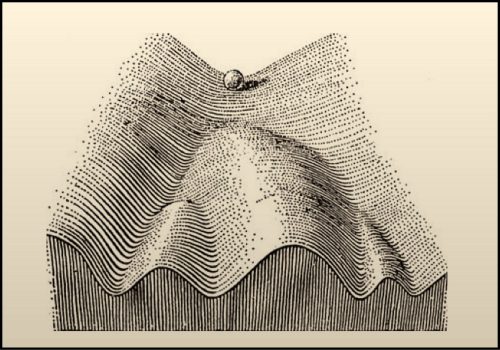
I also like how researchers of times past thought about development. The Epigenetic Landscape Model by C.H. Waddington is one of my favourite examples. To represent the process by which individual cells make fate decisions, he proposed that a cell can take different paths during development with each path representing a journey towards a unique fate. He drew a diagram to represent this idea – in his drawing a ball rolling down a mountainside presented a cell on its way to adopting a final fate. This drawing and the concepts that it represents is very inspiring to me. One can think about how to push cells developmentally down different trails or how to push the cells uphill (de-differentiation) and then down another trail (fate reassignment). For my own work, I try to think of the ball not as a single cell. Instead, to me it represents the entire eye-antennal disc. My lifetime goal is to figure out how the eye-antennal disc is guided down the mountain and how it ultimately gives rise to five distinct tissues and organs.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
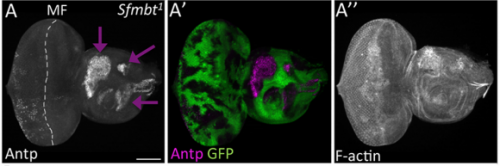
JZ Yes. For a long time, I couldn’t figure out why the eye disc adopts a wing fate but not any other tissue fates. I know it is likely due to the de-repression of Antp in the developing eye when PcG activity is impaired. However, why is Antp being activated when the epigenetic silencers are removed? This question was solved when a piece of data came back from another ongoing project, in which I found that over-expression of teashirt (tsh) was able to rescue the headless phenotype of Pax6 double mutant (Zhu et al, 2017). In those flies, little wings or thoracic bristles were found in the rescued head cuticle, so I wonder the endogenous expression of tsh might be the transcriptional activator of Antp when Pc is removed from the eye disc. I did an experiment to knockdown Pc and Tsh (Figure 3) at the same time and it turn out to be true. Tsh is required to activate Antp during the fate transformation. Although we do not know whether Tsh directly turns on Antp transcription or not, but at least we found some underlying mechanisms of the homology between the eye and the wing. I think the lesson here is to have multiple projects going on at the same time.
And what about the flipside: any moments of frustration or despair?
JZ For me, the frustration in this project is that isolating eye discs at early stages, such as in 1st and early 2nd larval instar, is almost impossible. Thus, we couldn’t verify our final model of PcG proteins being recruited to the genome at these specific stages using ChIP-Seq. I hope the techniques will advance and allow us to reveal how exactly these epigenetic regulators function in vivo.
What next for you Jinjin – I hear you’ve moved to Harvard?
JZ Yes, for my postdoctoral training, I will work with Dr. Robert Kingston, focusing on how PcG proteins control gene expression in mammalian system. The PcG proteins in mammals are much more complicated than in Drosophila. Different PcG complexes have multiple variants and each protein have multiple homologs. Thus, a diversity of possible mechanisms that might be used to generated a repressive state of gene expression, such as histone modification, chromatin compaction and higher-order genome organization. I think the eye-to-wing transformation project has brought me here, but I want to learn more about the underlying mechanisms of epigenetic regulators on the molecular level for my future research.
Where will this work take the Kumar lab?
JK My research group has started studying how the interplay between transcriptional networks and epigenetic complexes such as Polycomb, Trithorax, and SAGA controls fate specification within the eye-antennal. These studies build upon the findings of our paper described here in Development in which Pax6 and PcG proteins cooperate to repress wing fates from being adopted in the developing eye. Currently, we have evidence that Pax6 cooperates with Trithorax and SAGA complexes to control the number of antennae that are produced. We also have evidence that these same complexes work together to prevent the duplication of the entire eye-antennal disc. I am very excited about these preliminary findings and I think my lab, for the near future, will be focused on using the tissues within the eye-antennal disc to revisit several very basic questions – how does an organism control the fate, number, and placement of all of the organs that it needs for survival.
Finally, let’s move outside the lab – what do you like to do in your spare time?
JZ I travel with my husband during holidays and we have been to many national parks in US. We both like photographing wild animals. At home, I usually play piano and computer games if I don’t need to collect fly embryos.
JK When I am not in the lab or my office I enjoy playing tennis. It is a terrific sport and a good outlet to release stress. I try to mix tennis and work as well whenever possible. When I am at home working on a paper or grant, I have the Tennis Channel on in the background. If there is an important match, I will stream it on my work computer as well. I also take my camera wherever I go and enjoy photographing wild life and outdoor scenery.
Polycomb group (PcG) proteins and Pax6 cooperate to inhibit in vivo reprogramming of the developing Drosophila eye. Jinjin Zhu, Alison J. Ordway, Lena Weber, Kasun Buddika, Justin P. Kumar.
This is #39 in our interview series. Browse the archive here.


 (No Ratings Yet)
(No Ratings Yet)