YEN does it again
Posted by Vicki Metzis, on 29 June 2016
Last month saw the return of the Young Embryologist Network annual meeting held this year at the UCL Institute of Child Health. To settle into the long weekend, a number of us from the Briscoe Lab at the Crick Mill Hill site headed on down to central London to spend the day being inspired by talks from PhD students, Postdocs and several invited guest speakers. We were met with an impressive array of speakers, highly interactive poster sessions and an atmosphere buzzing with young embryologists demonstrating their clear passion for cell and developmental biology. With such excellent organisation and enthusiasm, we are happy to write about our thoughts of the meeting, and greatly anticipate the next event.
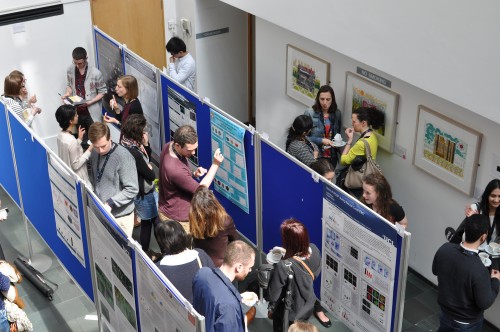
To kick off the day, our newest lab member, Teresa Rayon, opened the “Cell Fate Determination” session. Presenting her PhD project, Teresa demonstrated the importance of enhancer usage and developmental pathways that direct tissue-specific expression of Cdx2 in the early mouse blastocyst. Following this tour de force, the opening session only continued to satisfy our appetite for developmental biology, concluding with the two prize-winning talks of the day. Sarah Bowling (Rodriguez Lab), presented an elegant story on the role of mTOR signalling, a main component involved in cell competition in early embryos. Her studies showed the link between developmental signalling, metabolism and cell survival, for which she received the Sammy Lee Memorial Medal. This award recognises an early stage career embryologist, who not only carries out inspiring work but also has the ability to communicate their science in a personable manner, a skill that Sammy Lee was well known for. Following this, the session concluded with Sarah Harrison (Zernicka-Goetz lab), who was awarded a second place prize for her work on organoids, and how asymmetry occurs in the early embryo. At this point we were left with a clear feeling of excitement that in vitro systems such as stem cells have afforded developmental biologists, as more and more becomes possible at the tissue and organ level….
The second session of the day concentrated on polarity and asymmetry with the best place to focus being the zebrafish brain. The talks up until this point all had one thing in common: stunning imagery, which gave a natural build up into the first external speaker of the day, Dale Moulding from UCL Institute of Child Health. His talk covered new and emerging technologies in microscopy, covering a range of approaches from micro-CT, to OPT, SPIM and light-sheet imaging – a veritable repertoire of optical imaging solutions, which are continually pushing the boundaries of image resolution and depth of field.
After the lunch break the second guest session was filled with inspiring presentations on early embryonic development from Kathy Niakan (Francis Crick Institute) and Shankar Srinivas (University of Oxford). Kathy discussed mouse and human blastocyst development, focusing on the key transcriptional regulators involved in early lineage commitment. Shankar then followed with an examination of the cellular mechanisms involved in driving early AP patterning in the mouse, and in establishing a beating heart. Echoing earlier sentiments of the day, he demonstrated how light-sheet microscopy can be exploited to reveal impressive cellular-level detail in the context of whole organisms as they develop in real time. The generation of this type of live imaging data now poses new challenges to the developmental biology field, in how best to reconstruct individual cells and extrapolate the intricate details of developing organs. Such a feat will undoubtedly rely on the continued collaboration between biologists and physicists alike to develop the best possible data analysis solutions.
The final session, “Morphogenesis, Repair and Regeneration” had talks on cell matrix adhesions during contact inhibition of locomotion as well as cell movement in distal neuropore closure. To complete the session, Tapan Papilia (Hughes lab) gave an insight into Pax7 stem cells in muscles and their roles in regeneration and repair.

Closing the talks for the day, we welcomed Professor Paul Martin (University of Bristol), invited to give The Sammy Lee Memorial Lecture. Capturing the essence of YEN, Paul’s talk reminded us that it’s not only important to work hard, but to enjoy the work that you’re doing – an important aspect that can drive discovery. His captivating presentation highlighted that no matter what area of development you may find yourself in, the basic underpinnings of biology can have far reaching implications. Some of these are being realised in the field of tumour biology, where studies that began in fly have transitioned to vertebrate models of wound healing that are informing cancer treatments. Never knowing exactly where one will end up is definitely an attraction for many developmental biologists, and a motivation that drives us to work long (and sometimes tedious) hours at the bench or desk.

From cells to embryos, the YEN meeting makes a compelling case that the UK harbours many talented young scientists. Importantly, these individuals come from across the globe and are fuelled by a potent combination of excellent research leaders, high quality facilities and resources – a tradition that we are proud to be a part of now and into the future. An important conclusion that we take away from this meeting is that a successful “young embryologist” is in our view not only a creative, open-minded, cross-disciplinary individual, but also an excellent communicator, both to the public and to scientists alike. Events such as these are important to promote these aspirations. For this, we would like to thank the organising committee and sponsors who enabled this excellent meeting. We look forward to continuing our participation in future events organised by the young embryologists network and strongly encourage you to do the same.
Katherine Exelby & Vicki Metzis
For another view on YEN 2016, read Thomas’ report


 (7 votes)
(7 votes)
 Notch signalling regulates various aspects of vertebrate cartilage development, and Hilton and colleagues now
Notch signalling regulates various aspects of vertebrate cartilage development, and Hilton and colleagues now  protein Scribble is critical in establishing apical-basal polarity during epithelial development. Muthuswamy and coworkers now
protein Scribble is critical in establishing apical-basal polarity during epithelial development. Muthuswamy and coworkers now  Haptotaxis is directional cell migration in response to a gradient of substrate-bound cues. Bear and colleagues
Haptotaxis is directional cell migration in response to a gradient of substrate-bound cues. Bear and colleagues 
 De novo generation of photoreceptor cells is therapeutically promising for patients with retinal degenerative diseases. Seko and colleagues
De novo generation of photoreceptor cells is therapeutically promising for patients with retinal degenerative diseases. Seko and colleagues  pigmentation is an established model system for developmental patterning. Irion and colleagues
pigmentation is an established model system for developmental patterning. Irion and colleagues 
 identify
identify Pera and co-workers
Pera and co-workers  (2 votes)
(2 votes) (No Ratings Yet)
(No Ratings Yet)