We’ve all heard the aphorism that once you leave academia, you can’t go back. A little over two years ago, I wrote about my transition from academic research to scientific editing. Now, after completing my first year as an assistant professor of Biology at Reed College, I’m offering proof that lane changes, U-turns, and detours can lead you back to the ivory tower. Many people suggested that I wouldn’t be able to come back to academia when I chose to accept an editorial job at Cell, and I had every intention of sticking with my chosen path of scientific editing. After almost a year of sitting at a desk in an office that didn’t smell like acetic acid or freshly poured LB agar, a niggling feeling kept telling me that something wasn’t quite right. A few soul-searching diary entries, a couple of personality type indicators, and many conversations with friends and mentors helped me discern exactly what that feeling was trying to tell me. Scientific careers can follow many different trajectories and sometimes the “best fit” path is not always the easiest or most logical choice.
It is my hope that sharing my journey thus far helps scientists at all stages of their careers recognize that many valid career paths exist and that lane changes, U-turns, and detours are always possible. And I hope that this piece will spark conversation, here and elsewhere, about the varied and multiple careers scientists pursue.
The exit ramp
When I made the decision to leave my post-doc for the position at Cell, I focused on what the job offered: the ability to do more of what I liked – thinking and communicating science – and less of what I didn’t like – worrying about funding and dealing with the pressure to publish in high-impact journals. (The irony of working at a high-impact journal was not lost on me.) Plus the editorial job put me in the same time zone as the rest of my family. It was a logical and rational decision.
The novelty of scientific editing excited me in a way that my day-to-day work as a post-doc didn’t and, as a result, I disregarded the things that I actually enjoyed about working in academia, like the experimental troubleshooting, the microscopy, the competition to figure something out before someone else, the pipetting, the interactions with students. Working as an editor helped me hone my critical thinking skills, gave me a strong foundation in written communication, and also helped me clarify who I was, what I wanted, and what I valued. I need to make (or at least feel like I’m making) unique and creative contributions to science.
Lost without a map
In an era of tight funding and high-stakes research, my decision to leave a very good and relatively secure job for one fraught with stress and insecurity might seem a little odd, and certainly not very practical. It is, in fact, one of the least rational decisions I’ve made. And although we scientists are often known for our logic and careful reasoning, many of us are intuitive thinkers, making decisions based, at least partly, on gut instinct. This is not to say that I did not enjoy my time as an editor, because I did. I count myself incredibly fortunate to have worked as a scientific editor at Cell, and had my academic job search been unsuccessful, I’d still be handling manuscripts, recruiting papers, talking with authors, attending meetings as an editor, and savoring the opportunity to read papers and work with really smart people.
The U-turn…
Applying for assistant professorships while also working as a full-time editor forced me to focus on exactly what I wanted. While many of my post-doc friends and colleagues indiscriminately applied to 30 or 40 positions, I applied to seven. I was mindful of location (after living in a variety of places up and down the East Coast and in London, my husband and I wanted a place that had plenty of green space and the option to cycle and walk most places). I knew from multiple experiences that I wanted to work directly with undergraduate students and maintain a small, active research program. My weekends were dedicated to outlining my tentative research program and customizing my application materials for each position on my list. My scientific “U-turn” was possible, in large part, because of an incredibly generous post-doc mentor (Steve Wilson at UCL) who allowed me to resume some of the projects I’d left behind and use them as a jumping off point for my long-term research program.
The new path…
I’m not quite sure what the various search committees saw in my application, but I was extremely fortunate to have two fantastic offers to teach and do research at top-notch liberal arts schools. I love my current job at Reed College. My experiences as a high school science teacher (before grad school) and a scientific editor (before this position) endowed me with a unique and useful skillset. As a professor at small liberal arts college, I relish designing both curriculum and experiments. I enjoy reading, writing, and inviting notable scientist for seminars. I impact the future of science by teaching and mentoring undergraduates in my lab. I’ve embraced the academic lifestyle. And although our recently adopted dog, Mickey, would like to think his need for a run is what gets me up bright and early ~5am, it’s really the opportunity to share my love of research, encourage students to explore and experiment, and mentor budding scientists that get me out of bed every morning. The life of an assistant biology professor at a place like Reed can be incredibly rewarding, but it also comes with a healthy dose of stress. Fear of delivering a subpar lecture, apprehension about lab activities not working, and anxiety about my nascent research program certainly gave me some grey hairs. That said, I wouldn’t have traded this past year for anything (well, maybe more sleep). I’ve never learned as much, slept as little, worried as much, or felt as happy as I have this past year. And I wouldn’t have survived this year without the stress relief and support that my dog, husband, wonderful colleagues, mentors, and friends provided.
Even if Reed isn’t my final academic home, my journey so far has taught me to seize the opportunities that come my way, to learn and live as fully as I possibly can. And I hope that this one example of leaving and returning to academia gives you the courage to search for the career path that is right for you.
What are your experiences? Have you found your “dream job”? What have you learned from taking one of the less mainstream career paths? Please post here to continue this exploration of career trajectories.
 (46 votes)
(46 votes)
 Loading...
Loading...
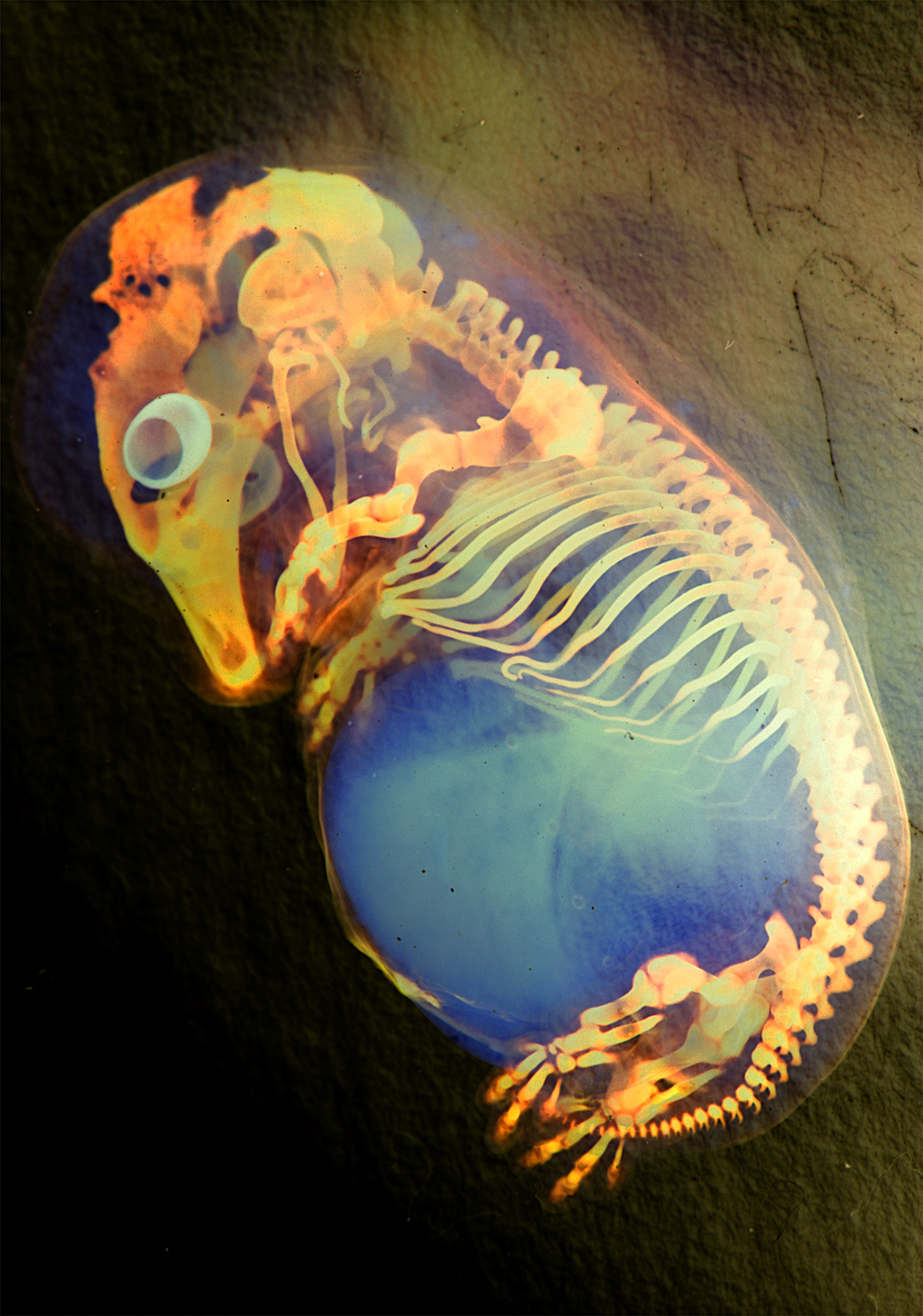 This great picture was taken by Marina Venero Galanternik (University of Utah), Rodrigo G. Arzate-Mejía (Universidad Nacional Autonoma de Mexico), Jennifer McKey (Universite Montpellier) and William Munoz (The University of Texas MD Anderson Cancer Center). It shows a colour inverted image of a skeleton preparation of a pig (Sus scrofa domesticus) embryo.
This great picture was taken by Marina Venero Galanternik (University of Utah), Rodrigo G. Arzate-Mejía (Universidad Nacional Autonoma de Mexico), Jennifer McKey (Universite Montpellier) and William Munoz (The University of Texas MD Anderson Cancer Center). It shows a colour inverted image of a skeleton preparation of a pig (Sus scrofa domesticus) embryo.

 (2 votes)
(2 votes) (46 votes)
(46 votes) (No Ratings Yet)
(No Ratings Yet)







