Here are the highlights from the current issue of Development:
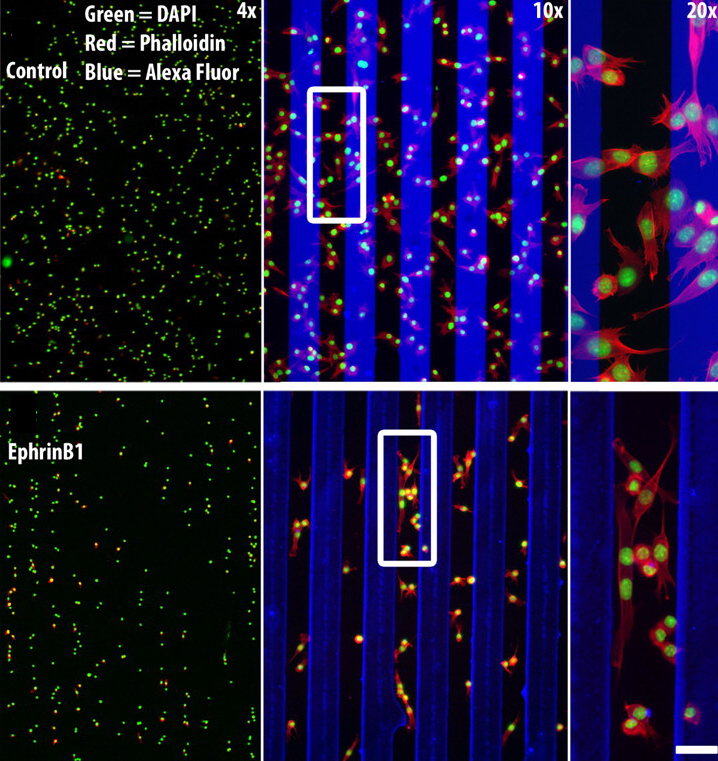
Balanced ephrin/Eph signals drive topographic mapping
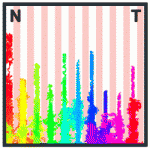
During development of the retinotectal axonal projection, which connects the retina to the optic tectum in the midbrain, the axons of neighbouring retinal ganglion cells project to neighbouring positions in the optic tectum (topographical mapping). However, although retinal fibres rigidly target their destinations in some experimental circumstances, in others they adapt to grossly diverse targets. Here (see p. 335), Franco Weth and colleagues present a surprisingly simple model that explains these hitherto puzzling discrepancies. In this model, topographical axonal mapping relies solely on the balance of forward and reverse signalling by the ephrin/Eph family of guidance molecules. To test their model, the researchers develop a novel ephrin/Eph (double-cue) stripe assay and show experimentally that the simultaneous presence of forward and reverse ephrin/Eph signalling is indeed sufficient for appropriate topographic growth decisions in chick embryonic nerve fibres. Moreover, using computer simulations, they show that their new model is capable of reproducing the discrepant data collected over the years on topographic mapping by the retinotectal axonal projection.
Complex dance of eye morphogenesis unveiled
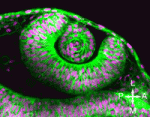
Optic cup morphogenesis (OCM), which generates the basic structure of the vertebrate eye, is usually depicted as a series of epithelial sheet folding events but experimental evidence to support this stepwise model is lacking. Now, Kristen Kwan, Chi-Bin Chien and colleagues investigate the cellular dynamics of OCM in zebrafish by combining four-dimensional time-lapse imaging and cell tracking (see p. 359). The researchers show that OCM depends on a complex set of sometimes unanticipated cell movements that are coordinated between the prospective neural retina, retinal pigmented epithelium and lens, the tissues that comprise the mature optic cup. Using their cell tracking data, the researchers construct subdomain fate maps for these three tissues that might provide clues to developmental signalling events. Finally, they show that similar movements occur during chick eye morphogenesis, which suggests that the complex choreography of cell movements that shape the vertebrate eye is conserved. These new insights into eye development could, therefore, help to improve our understanding of human eye defects.
Shedding light on Rho kinase signalling
Small-molecule inhibitors can be used as loss-of-function tools to investigate the molecular mechanisms of development but, although exposure to these inhibitors can be temporally controlled, their effects are not spatially restricted. Now, Nanette Nascone-Yoder and colleagues have generated a pharmacological agent that allows for photoactivatable, and hence spatiotemporally limited, inhibition of Rho kinase (see p. 437). Rho signalling is involved in many morphogenetic events, including primitive gut elongation in Xenopus embryos. The researchers install a photolabile ‘caging’ group on Rockout, a small-molecule inhibitor of Rho kinase, and show that caged Rockout (cRO) can permeate Xenopus embryonic tissues. When cultured in the dark, cRO-treated embryos develop normally, but UV irradiation of the right side of these embryos produces animals with a unilaterally shortened gut. Finally, the use of cRO reveals a differential requirement for Rho signalling on the left and right sides of the gut during intestinal rotation. Photocaging pharmacological inhibitors, the researchers conclude, might be a generalisable technique for producing loss-of-function reagents for use in multiple developmental contexts.
The eyes have it: bioelectric induction
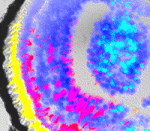
Endogenous steady-state ion currents, voltage gradients and electric fields produced by ion channels and pumps regulate patterning and have been implicated in adult eye wound healing. So might they play a role in eye development? On p. 313 Michael Levin and co-workers report that transmembrane voltage potential (Vmem) is an important component of the eye induction cascade in Xenopus. The researchers identify a hyperpolarised cluster of cells in the anterior neural field of Xenopus embryos and show that depolarisation of the lineages from which these cells are derived results in malformed eyes. Remarkably, given our understanding of lineage restrictions and plasticity, manipulation of Vmem in non-eye cells induces ectopic eye formation far outside the anterior neural field. Other experiments show that a Ca2+ channel-dependent pathway transduces the Vmem signal and regulates the pattern of eye field transcription factor expression. This new information on the roles of voltage gradients as mediators of patterning during embryogenesis might have implications for the development of regenerative approaches to ocular diseases. (See also this post on the Node.)
Heads up for neural specification
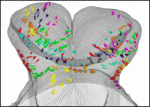
During mouse embryogenesis, the anterior ectoderm develops into neural derivatives (the forebrain) and non-neural derivatives (the cephalic non-neural ectoderm). On p. 423, Kirstie Lawson, Anne Camus and co-workers use single-cell labelling and gene expression analysis to provide new insights into this cell fate choice. At late gastrulation, they report, the expression patterns of anterior ectoderm genes overlap significantly and correlate with areas of prospective fate but do not define lineages. They show that the rostral limit to forebrain contribution is more distal than previously reported. Finally, they report that some precursors of the anterior neural ridge, a signalling centre that is involved in forebrain development and patterning, are clonally related to neural ectoderm and are dispersed over a broad area of the anterior ectoderm where neural precursors also reside. Together, these results suggest that, although the segregation of neural and non-neural cells in the anterior ectoderm is incomplete at the gastrulation stage, there are elements of regionalisation in this tissue that preconfigure the organisation of the head.
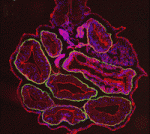
ROCK solid epithelial organisation
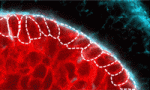
The basement membrane is essential for epithelial tissue organisation and function but what restricts the basement membrane to the basal periphery of epithelial tissues and what are the basement membrane-mediated signals that regulate coordinated tissue organisation? On p. 411, Melinda Larsen and colleagues use cultures of embryonic mouse submandibular salivary glands to investigate these questions. They show that inhibition of the Rho kinase ROCK in these cultures results in basement membrane accumulation throughout the epithelial compartment. ROCK-mediated control of Par-1b localisation in the outer basal epithelial cell layer (which produces basement membrane) is responsible for normal basal basement membrane positioning, they report. Moreover, inhibition of Par-1b kinase activity prevents basement membrane deposition and disrupts tissue organisation. Conversely, overexpression of Par-1b drives ectopic basement membrane production. These and other results suggest that Par-1b is a master regulator of basement membrane deposition in developing salivary glands and that ROCK control of Par-1b function is essential for normal epithelial integrity and organisation.
PLUS…
When cell cycle meets development
The recent Company of Biologists workshop ‘Growth, Division and Differentiation: Understanding Developmental Control’, which was held in September 2011 at Wiston House, West Sussex, UK. Kaldis and Richardson review the common themes that emerged from the meeting, highlighting novel insights into the interplay between regulators of cell proliferation and differentiation during development.
See the Meeting Review on p. 225
Palatogenesis: morphogenetic and molecular mechanisms of secondary palate development
Mammalian palatogenesis is a highly regulated morphogenetic process, the complexity of which is reflected by the common occurrence of cleft palate in humans. Here, Bush and Jiang review major advances in our understanding of the mechanisms that control secondary palate development.
See the Review Article on p. 231
Shaping sound in space: the regulation of inner ear patterning
Groves and Fekete review recent studies of inner ear development and patterning, which reveal that multiple stages of ear development are orchestrated by gradients of signaling molecules.
See the Review Article on p. 245
 (No Ratings Yet)
(No Ratings Yet)
 Loading...
Loading...
 eurostemcell.org is multilingual! Or tri-lingual, at least.
eurostemcell.org is multilingual! Or tri-lingual, at least.

 Type 1 Diabetes: How could stem cells help?
Type 1 Diabetes: How could stem cells help?

 (2 votes)
(2 votes) Do we need another book on human stem cell biology? The field is fairly long in the tooth now, thirteen years after Thomson first derived human embryonic stem (ES) cells (Thomson et al., 1998). There are many books that cover the theoretical aspects of the discipline (Oderico et al., 2004) and others that attempt to collate protocols useful to human stem cell biologists (Sullivan et al., 2007). Nevertheless, the new book Human Stem Cell Technology and Biology succeeds in combining both of these characteristics, providing not only a clear account of the scientific discoveries underpinning human stem cell biology, but also a useful range of laboratory protocols. The end product may be less comprehensive than Lanza’s classic text (Lanza et al., 2009), but it is perhaps more manageable and appropriate for a newcomer to the field or for an early career scientist working with human pluripotent cell lines for the first time.
Do we need another book on human stem cell biology? The field is fairly long in the tooth now, thirteen years after Thomson first derived human embryonic stem (ES) cells (Thomson et al., 1998). There are many books that cover the theoretical aspects of the discipline (Oderico et al., 2004) and others that attempt to collate protocols useful to human stem cell biologists (Sullivan et al., 2007). Nevertheless, the new book Human Stem Cell Technology and Biology succeeds in combining both of these characteristics, providing not only a clear account of the scientific discoveries underpinning human stem cell biology, but also a useful range of laboratory protocols. The end product may be less comprehensive than Lanza’s classic text (Lanza et al., 2009), but it is perhaps more manageable and appropriate for a newcomer to the field or for an early career scientist working with human pluripotent cell lines for the first time. (No Ratings Yet)
(No Ratings Yet)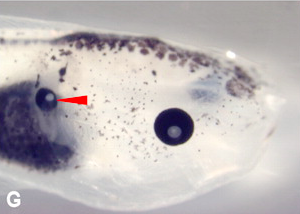 Ectopic eye, with lens, formed on a tadpole’s gut.
Ectopic eye, with lens, formed on a tadpole’s gut.






 One of the key goals of modern cell and developmental biology is to expose the underlying principles that drive cell differentiation and to elucidate how organisms construct functional multicellular structures. Thanks to advances in sequencing, high throughput screens and sophisticated imaging technologies, these fields are now awash with quantitative descriptions of gene transcription, cell signaling and cell mechanics. However, extracting key principles from the flood of new data is a major challenge for researchers and a central obstacle to fundamental progress in cell and developmental biology. The tools required to interpret this vast amount of biological data and to test hypotheses based on these studies can be found in quantitative analysis and mathematical modeling. With the book Mathematical Models of Biological Systems, Hugo van den Berg aims to contribute to the training of a new generation of biologists and mathematicians and to provide them with an introduction to the methods that are now available to quantitatively analyze biological data.
One of the key goals of modern cell and developmental biology is to expose the underlying principles that drive cell differentiation and to elucidate how organisms construct functional multicellular structures. Thanks to advances in sequencing, high throughput screens and sophisticated imaging technologies, these fields are now awash with quantitative descriptions of gene transcription, cell signaling and cell mechanics. However, extracting key principles from the flood of new data is a major challenge for researchers and a central obstacle to fundamental progress in cell and developmental biology. The tools required to interpret this vast amount of biological data and to test hypotheses based on these studies can be found in quantitative analysis and mathematical modeling. With the book Mathematical Models of Biological Systems, Hugo van den Berg aims to contribute to the training of a new generation of biologists and mathematicians and to provide them with an introduction to the methods that are now available to quantitatively analyze biological data. Dates for your calendar
Dates for your calendar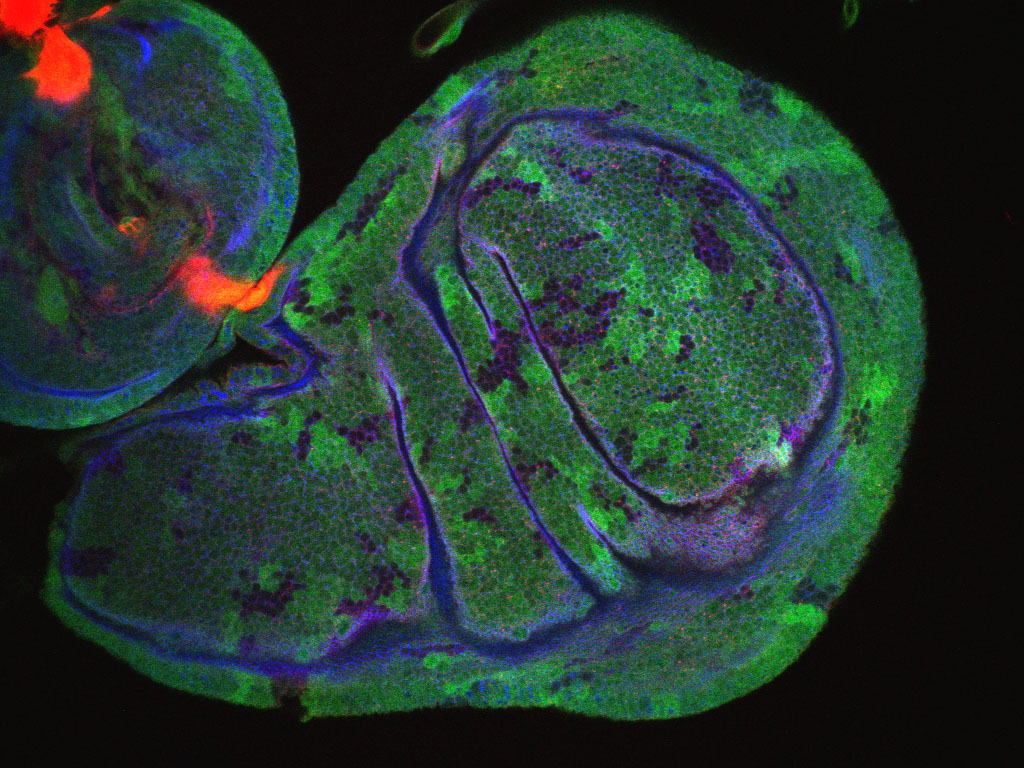
 Following the discovery of the structure of DNA and during the early days of molecular biology, RNA was considered to be a less interesting cellular component to study than DNA. This was primarily because RNA was thought to be simply a molecular photocopy of the genetic blue print stored in DNA. But how things have changed! Since those early days, our understanding of the cellular roles of RNA has changed radically. RNA is now considered to be of central importance to both molecular biology and cellular function. Far from only containing genetic information, RNA is now regarded to have credible catalytic properties through the availability of its 2′-OH, a reactive group that replaces a non-reactive ‘O’ atom in DNA. Moreover, its catalytic roles include key functions in the most important molecular machines of the cell, such as the spliceosome and ribosome. In hindsight, it would perhaps not be so surprising if the RNA world hypothesis turned out to be correct. This hypothesis states that the first life forms on our planet were RNA-based simple cells in the pre-biotic soup. RNA is certainly a better candidate than either DNA or proteins for a self-replicating molecule that acts both as a template for, and that has the necessary catalytic machinery to perform, its own replication. Moreover, the discovery that most mRNAs are spliced, and the gradual uncovering of a breathtaking number of ways in which gene expression is regulated post-transcriptionally, have meant that the field of RNA has undergone rapid growth in the past few decades. This rapid growth has recently increased even further because of the discovery of RNA interference (RNAi), as well as the discovery that small RNAs, distinct from tRNA and snRNA, undergo processing to fulfil a range of cellular functions. These include the regulation of transposable element transposition by piRNAs, regulation of translation by microRNAs and still poorly explored large non-coding RNAs (ncRNA). Many of these ncRNAs have turned out to have important roles in development and during disease processes, such as cancer. Therefore, it is clear that all aspects of RNA molecular biology have now become central to our understanding of cell and developmental biology.
Following the discovery of the structure of DNA and during the early days of molecular biology, RNA was considered to be a less interesting cellular component to study than DNA. This was primarily because RNA was thought to be simply a molecular photocopy of the genetic blue print stored in DNA. But how things have changed! Since those early days, our understanding of the cellular roles of RNA has changed radically. RNA is now considered to be of central importance to both molecular biology and cellular function. Far from only containing genetic information, RNA is now regarded to have credible catalytic properties through the availability of its 2′-OH, a reactive group that replaces a non-reactive ‘O’ atom in DNA. Moreover, its catalytic roles include key functions in the most important molecular machines of the cell, such as the spliceosome and ribosome. In hindsight, it would perhaps not be so surprising if the RNA world hypothesis turned out to be correct. This hypothesis states that the first life forms on our planet were RNA-based simple cells in the pre-biotic soup. RNA is certainly a better candidate than either DNA or proteins for a self-replicating molecule that acts both as a template for, and that has the necessary catalytic machinery to perform, its own replication. Moreover, the discovery that most mRNAs are spliced, and the gradual uncovering of a breathtaking number of ways in which gene expression is regulated post-transcriptionally, have meant that the field of RNA has undergone rapid growth in the past few decades. This rapid growth has recently increased even further because of the discovery of RNA interference (RNAi), as well as the discovery that small RNAs, distinct from tRNA and snRNA, undergo processing to fulfil a range of cellular functions. These include the regulation of transposable element transposition by piRNAs, regulation of translation by microRNAs and still poorly explored large non-coding RNAs (ncRNA). Many of these ncRNAs have turned out to have important roles in development and during disease processes, such as cancer. Therefore, it is clear that all aspects of RNA molecular biology have now become central to our understanding of cell and developmental biology.