Retracting sheaths and words
Posted by Alexandria Hughes, on 17 July 2020
My mentor, Bruce Appel, emphasizes the importance of communicating science clearly and precisely. Consequently, I have watched my peers and myself deliver ever-improving talks, posters, and manuscripts during our time in the lab. I think that many people in science appreciate that clear communication is essential for others to be able to interpret findings and effectively build upon what has been done. A corollary lesson that I wasn’t expecting to learn from our latest project, published recently in Nature Neuroscience (Hughes and Appel, 2020), is how imprecise language can muddle and confound understanding, obstructing progress.
Our lab studies oligodendrocyte lineage cells using zebrafish as a model system. In the central nervous system, oligodendrocytes wrap neuronal axons with myelin, a lipid-rich membrane that increases conduction velocity. Zebrafish are both genetically and optically accessible, allowing us to image cellular interactions during myelination in live larvae. A live-imaging experiment can catch oligodendrocytes extending numerous, arborizing processes that search for and begin to wrap axons with myelin membrane. Some of these nascent wraps continue to grow and mature, whereas other wraps appear to be eliminated. How are myelin sheaths eliminated?
Many structures that also are studied by live-imaging, like neuronal neurites, undergo similar deformations during development. Neurons elaborate and occasionally withdraw neurites, and this latter process is termed “retraction”. Oligodendrocytes generate processes that branch similarly to neurites. Like neurites, these processes withdraw, and these events have been described as “retraction”. But oligodendrocyte processes also do something very different than neurites: upon contacting a target axon, an oligodendrocyte process can deposit a reservoir of myelin lipids that spreads like a liquid droplet as the process begins wrapping the axon (Nawaz et al., 2015). Can fluid-like myelin sheaths, like the simple processes that gave rise to them, also be withdrawn? Live confocal microscopy doesn’t show us sheaths unraveling or processes reeled in by the cell body. Instead, sheaths merely reduce in size and disappear. By using the word “retraction” to describe all oligodendrocyte process disappearances, an untested mechanism was invoked to account for all myelin sheath elimination.
If myelin sheaths aren’t retracted, what alternative mechanisms could remove sheaths? Many structures in the developing nervous system, including synapses, neuronal precursors, and neurons, are overproduced and can be pruned by microglia, the resident immune cell type of the CNS. We had previously found a number of similarities between the formation of myelin sheaths and neuronal postsynapses (Hughes and Appel, 2019), raising the possibility that these structures might also be eliminated similarly.
Admittedly, I spent a lot of time thinking about microglia before they constituted a reasonable suspect in sheath elimination. Early in grad school, I had read a paper that found that microglia regularly survey the zebrafish spinal cord and quickly swoop in to clear laser-ablated neurons (Morsch et al., 2015) and I was really curious to see if these cells interact with oligodendrocytes during normal development. Something I particularly like about working with zebrafish is how easy it is to casually pursue these types of side-curiosities. I lost no time to generating the construct to label microglia, because I did it in parallel with other cloning I needed to do; I injected the construct to generate a microglia reporter transgenic line after I had performed my priority injections for experiments that week. A few months later, I had a microglia reporter line and was ready to find out if microglia and oligodendrocytes interact during myelination.
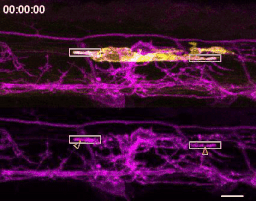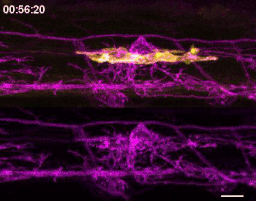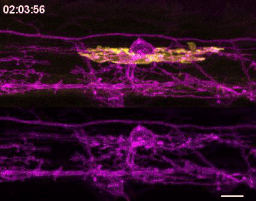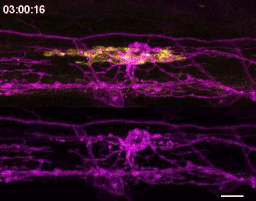
The first time I timelapse imaged microglia interacting with myelin, to my surprise and delight I found microglia engulfing nascent myelin sheaths. I presented a video at lab meeting and was encouraged by the enthusiasm and questions raised by my labmates. How many microglia are there and how many sheaths are they eating? Do oligodendrocytes die when their sheaths are eaten? What regulates sheath phagocytosis? These tractable questions started to crystallize the phenomenon into a project.
At this stage, our recognition that myelin sheath development shares numerous similarities with synapse development was pivotal. Work by Dorothy Schafer, Beth Stevens, and Marie-Ève Tremblay had previously shown that microglia contact and phagocytose synapses in a neuronal activity-regulated manner (Schafer et al., 2012; Tremblay et al., 2010). Inspired by this work, the hypothesis and predictions that would form the foundation of the project emerged by analogy. Like synapses, do microglia phagocytose myelin in an activity-regulated manner? Will preventing pruning cause extra myelin to persist? With a path laid out, experiments moved swiftly. We found that microglia do survey and phagocytose myelin sheaths, and neuronal activity spurs microglia to trade-off between interacting with neuronal somas and phagocytosing myelin from myelinated axons. We further found that this program is robust enough that blocking it (via microglial ablation) caused excessive and ectopic myelin to develop.
We wrote up and submitted a much shorter version of our paper, concurrent with deposition to the preprint server BioRxiv, in summer 2019. In review, it became clear that microglia-mediated myelin pruning was incompatible with our field’s understanding that sheaths are solely eliminated by retraction. At first, I saw this as a purely semantic problem: myelin sheaths disappear, and the word “retract” has been used to describe this observation but oversteps into a mechanism that hasn’t been tested. I was resistant to confronting retraction but was persuaded by a reviewer’s argument that it would be informative to know what fraction of sheath elimination is contributed by microglia. Figuring out how to quantify sheath loss in an unbiased way took longer than any other experiment in the paper and pushed my image analysis forward in new ways. These data were the very last addition to the paper, but they appear inconspicuously in the middle of Figure 2! Ultimately, we found that sheaths are both phagocytosed by microglia and disappear independently of microglial contact, with phagocytosis accounting for the majority of sheath loss. This latter, microglia-independent category of lost sheaths might be called “retraction”, as we do in the paper.
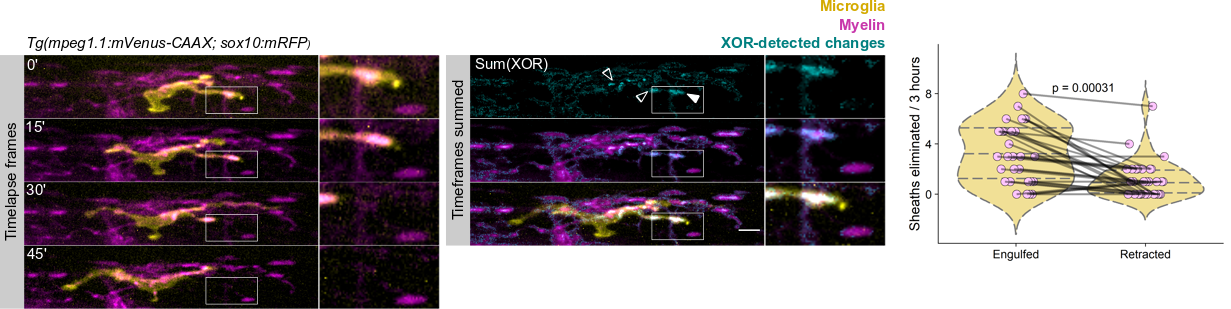
I still have some reservations about “retraction”. Prior to labeling microglia, we accepted that sheaths were solely eliminated by retraction: when oligodendrocytes were the only cell type visible, it was easy to grant cell autonomy to all visible changes and to forget that other cell types are lurking in the dark. Similarly, it’s possible that additional unlabeled cell types might engulf the microglia-independent subset of disappearing sheaths.
References
Hughes, A. N. and Appel, B. (2019). Oligodendrocytes express synaptic proteins that modulate myelin sheath formation. Nat. Commun. 10, 4125.
Hughes, A. N. and Appel, B. (2020). Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci. in press.
Morsch, M., Radford, R., Lee, A., Don, E. K., Badrock, A. P., Hall, T. E., Cole, N. J. and Chung, R. (2015). In vivo characterization of microglial engulfment of dying neurons in the zebrafish spinal cord. Front. Cell. Neurosci. 9, 321.
Nawaz, S., Sánchez, P., Schmitt, S., Snaidero, N., Mitkovski, M., Velte, C., Brückner, B. R., Alexopoulos, I., Czopka, T., Jung, S. Y., et al. (2015). Actin Filament Turnover Drives Leading Edge Growth during Myelin Sheath Formation in the Central Nervous System. Dev. Cell 34, 139–151.
Schafer, D. P., Lehrman, E. K., Kautzman, A. G., Koyama, R., Mardinly, A. R., Yamasaki, R., Ransohoff, R. M., Greenberg, M. E., Barres, B. A. and Stevens, B. (2012). Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 74, 691–705.
Tremblay, M.-È., Lowery, R. L. and Majewska, A. K. (2010). Microglial Interactions with Synapses Are Modulated by Visual Experience. PLoS Biol. 8, e1000527.


 (4 votes)
(4 votes)