In Development, Vol 137 (Issue 14)
Posted by Seema Grewal, on 23 June 2010
Here are the research highlights from the current issue of Development. You can find these on the Development site but we thought it would be useful to have them posted on the Node, too.
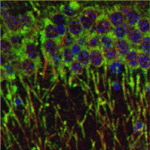
Brainy signals for actin dynamics
During brain development, neurite outgrowth and neuronal migration establish the brain architecture needed for brain function. Now, Eric Olson and colleagues reveal a regulatory feedback loop that links the cytoskeletal changes that provide the mechanical force needed for neurite outgrowth and migration to nuclear gene transcription during mouse brain development (see p. 2365). Myocardin-related transcription factors (MRTFs), the expression of which is forebrain enriched, translocate to the nucleus in response to actin polymerisation and cooperate with serum response factor (Srf) to regulate the expression of cytoskeletal genes. The researchers show that either Mrtfa orMrtfb is sufficient to support brain development but that the brain-specific deletion of both produces brain abnormalities similar to those caused by Srf deletion. These abnormalities, they report, are accompanied by dysregulation of the actin-severing protein gelsolin and of the kinase Pctaire1, which cooperates with Cdk5 to initiate a kinase cascade that governs cytoskeletal rearrangements. The researchers suggest, therefore, that MRTFs couple two signalling pathways that modulate cytoskeletal dynamics during neurite outgrowth and neuronal development.
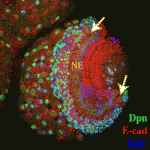 Proliferation’s not over ’til the Fat-Hippo sings
Proliferation’s not over ’til the Fat-Hippo sings
During development, transitions from proliferating, undifferentiated cells to quiescent, differentiated cells are tightly regulated to ensure that organs reach the correct size. Kenneth Irvine and colleagues now reveal that Fat-Hippo and Notch signalling influence this important transition during optic lobe development in Drosophila (see p. 2397). Like the vertebrate nervous system, the Drosophilaoptic lobe develops from neuroepithelial cells, which function as symmetrically dividing neural progenitors. The Fat-Hippo signalling pathway, which contains the large cadherin Fat and the serine/threonine kinase Hippo, regulates the transcription of cell proliferation and survival genes. The researchers report that neuroepithelial cells in the Drosophila optic lobe undergo a cell-cycle arrest that is regulated by Fat-Hippo signalling before converting to neuroblasts. They also identify a role for Notch signalling in committing neuroepithelial cells to become neuroblasts. These and other results suggest that, by arresting the cell cycle, Fat-Hippo signalling contributes to the accumulation of Delta, which modulates Notch signalling and triggers neuroepithelial differentiation. A similar mechanism might be involved in vertebrate neural development.
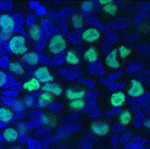 Pluripotent stem cell derivation gets a (2i-)LIFt
Pluripotent stem cell derivation gets a (2i-)LIFt
Pluripotent mouse embryonic stem (ES) cells are obtained directly from the mouse epiblast, while pluripotent embryonic germ (EG) cells can be derived from unipotent mouse primordial germ cells (PGCs) by epigenetic reprogramming. But how similar are EG and ES cells? On p. 2279, Azim Surani, Austin Smith and colleagues report that these cells share a conserved molecular and developmental ‘ground state’. ES cells can be established using the cytokine LIF combined with the inhibition of GSK3 and of mitogen-activated protein kinase signalling (so-called 2i-LIF culture). The researchers show that pluripotent mouse EG cells can also be efficiently established using 2i-LIF culture. Then, using the same conditions, they derive rat EG cells for the first time. These cells express similar markers to rat and mouse ES cells, they report, and can contribute extensively to chimeric rats. Together, these findings raise the possibility that 2i-LIF culture could be used to derive EG cell lines with pluripotent ground state properties from other species, including humans.
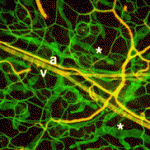 Integrin complexity at the heart of angiogenesis
Integrin complexity at the heart of angiogenesis
Integrin cell adhesion receptors and their ligand fibronectin play important roles during disease-associated and developmental angiogenesis. However, there are many different integrins, each of which contains a specific α subunit and a specific β subunit, and it is not clear which α subunits are involved in angiogenesis. Now, Arjan van der Flier and colleagues implicate both α5 and αv integrins, the major endothelial fibronectin receptors, in vascular remodelling during mouse development (see p. 2439). The researchers first show that, unexpectedly, the endothelial-specific knockout of α5 integrin has no obvious effect on developmental angiogenesis. They then test whether αv integrins compensate for the absence of α5 integrins by generating endothelium-specific α5; αv double-knockout mice. Vasculogenesis and angiogenesis are initially normal in these mice, but subsequent remodelling defects in the great vessels and the heart eventually cause embryonic death. Further investigations into the complex interplay among integrins during angiogenesis revealed here could lead to the development of effective anti-angiogenic drugs for cancer therapy.
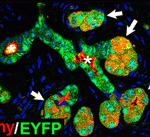 Pancreatic plasticity: a mixed message
Pancreatic plasticity: a mixed message
The pancreas is a dual function organ. Acinar cells in the exocrine gland make digestive enzymes, which enter the intestine through ducts, while the islets of Langerhans in the endocrine gland make insulin and other hormones, which enter the blood system directly. However, insulin-producing (insulin+) cells also appear in the hyperplastic ducts that develop in chronic pancreatitis and in pancreatic cancer, which begs the question: do hyperplastic ductal cells and their associated insulin+cells have a common origin? On p. 2289, Anna Means and colleagues use genetic lineage tracing and a mouse model that develops insulin+-cell-containing hyperplastic ducts in response to growth factor signalling to answer this question. They report that the hyperplastic ductal cells arise largely from the transdifferentiation of acinar cells. However, the insulin+ cells adjacent to the hyperplastic ducts arise from pre-existing insulin+ cells that intercalate into the ducts as they develop, rather than from insulin+ cell neogenesis, a result that has implications for efforts to treat diabetes through β cell regeneration.
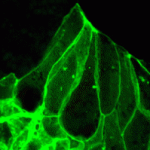 MID-way to neural tube closure
MID-way to neural tube closure
During vertebrate neural tube (NT) closure, the neural plate bends and fuses to form the hollow structure from which the CNS develops. Although neuroepithelial cell elongation and apical constriction underlie this morphological process, the role played by cytoskeletal elements in these cell shape changes is poorly understood. On p. 2329, however, Naoto Ueno and co-workers report that the Xenopusorthologues of human MID1 (which encodes the TRIM protein midline 1 and which is responsible for Opitz G/BBB syndrome) and MID2 (a MID1paralogue) regulate microtubule stabilisation during Xenopus NT closure. The researchers show that knockdown of Xenopus MIDs (xMIDs) disrupts epithelial morphology in the neural plate and leads to NT defects. This abnormal phenotype, they report, is caused by microtubule destabilisation and disorganisation in neuroepithelial cells. Furthermore, xMID knockdown disrupts the morphogenesis of other epithelial organs. Thus, microtubule regulation by the MIDs seems to be crucial for several epithelial remodelling processes, which might explain the developmental abnormalities seen in Opitz G/BBB syndrome.


 (No Ratings Yet)
(No Ratings Yet)