Ancient origin of the vertebrate sympathetic nervous system
Posted by BEdens, on 1 June 2024
A recent paper “Neural crest origin of sympathetic neurons at the dawn of vertebrates” challenges the prevailing dogma that the sympathetic ganglia arose only in jawed vertebrates. Instead, based on the findings in the sea lamprey, the authors suggest a late-developing rudimentary sympathetic nervous system may be present in the earliest jawless vertebrates. First author Brittany Edens and corresponding author Marianne Bronner tell us the story behind the paper.

Wild-caught mature ammocete sourced from the Great Lakes.
How did the project start?
Marianne: Brittany was doing some staining of lamprey larvae with different antibody markers with the goal of defining different types of neurons in the developing enteric nervous system. We got together to look over the data and realized that some of the neuronal staining was not in the gut but dorsal to the gut in a position that was appropriate for the sympathetic nervous system. This was a surprise since lamprey are not supposed to have a sympathetic nervous system. So we started looking for more markers to test this possibility more rigorously.
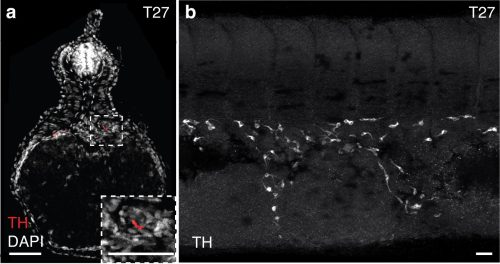
Detection of sympathoblasts in late embryonic lamprey: The catecholamine biosynthetic processing enzyme tyrosine hydroxylase (TH) is detected by immunohistochemistry at T27 in (a) transverse sections and (b) lateral whole-mount. TH+ sympathoblasts are localized dorsal to the yolk tube and flank the midline in bilateral streams. Scale bars=50mm (a) and 10mm (b).
Why do you think it’s been previously thought that jawless vertebrates lacked the sympathetic nervous system?
Marianne: That’s easy to answer. We think people (including ourselves) were initially looking at the wrong time. In higher vertebrates, the sympathetic nervous system develops rather early in development, initiating when neural crest cells begin to coalesce around the dorsal aorta. We actually looked at comparable stages in lamprey and did not see the markers characteristic of sympathetic neurons co-expressed. However, when we looked at larvae at about 1 month of development, we observed not only sympathetic marker genes but also the transcription factors known to be involved in their specification. Thus, there was a heterochrony in terms of the time of differentiation.
Why did you choose the lamprey to answer your questions?
Marianne: Lamprey are jawless vertebrates and have an important phylogenetic position at the base of the vertebrate tree of life. Lamprey fossils from the Cambrian period resemble modern lamprey in morphology. While we have no access to a “vertebrate ancestor” and lamprey have continued to evolve, they still are the closest approximation to what we think the ancestor may have looked liked.
Can you summarise the key findings of the paper in a paragraph?
Marianne: In gnathostomes (jawed vertebrates), the neural crest gives rise to a fate-restricted sympathoadrenal progenitor from which sympathetic neurons of the autonomic nervous system arise. A transcriptional program including Ascl1, Phox2b, and Hand2 specifies neural crest towards sympathoadrenal fates, and also promotes catecholaminergic identity (i.e., expression of tyrosine hydroxylase and dopamine beta-hydroxylase enzymes). Upon maturation, these neural crest-derived sympathetic neurons will express various pan-neuronal genes, as well as genes specific for catecholaminergic function. While the earliest vertebrates, which lacked jaws, were historically believed to lack sympathetic neurons within the trunk, we found evidence of these cells in the jawless vertebrate sea lamprey. We found that the same core transcription factors involved in sympathoadrenal specification were co-expressed in cells throughout the trunk in lamprey, as were the catecholamine pathway enzymes. Later in larval stages, these cells upregulated expression of pan-neuronal markers. Lineage tracing indicated a conserved origin in the trunk neural crest and finally, RNA-sequencing analysis suggested a transcriptional profile that was consistent with sympathetic neuron identity. Altogether our findings challenge the prevailing dogma that the sympathetic ganglia are a gnathostome innovation.
Were you surprised to find a rudimentary sympathetic nervous system in the lamprey?
Marianne: Yes indeed. We expected to see no sympathetic nervous system since that is what the literature says. It was a real surprise to see neurons in the right place with characteristics of sympathetic neurons.
How does the lamprey’s sympathetic nervous system differ from that in jawed vertebrates?
Marianne: There are many fewer neurons than seen in amniote embryos and no distinct ganglia. Just a few scattered cells all along the trunk region.
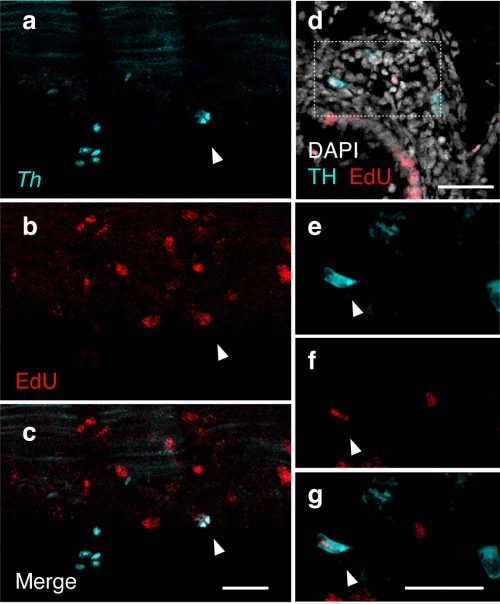
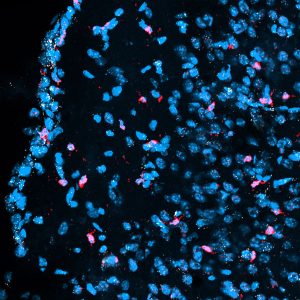
Ongoing proliferation of sympathoblasts in lamprey ammocetes: (a-c) HCR detection of Th (teal) and EdU staining (red) in ammocetes following an 8 hour EdU incubation. EdU detection in Th-expressing cells (indicated by arrowhead) reveals active division of sympathetic progenitors/neurons in lamprey trunk into ammocete stages. (d-g) Immunohistochemical detection of TH (teal) and EdU (red) co-expression in transverse sections of ammocetes following an 8 hour EdU incubation. Co-expression is denoted by arrowheads (n-p). DAPI is shown in white. Scale bars=50mm.
Brittany, were there any particular result or eureka moment that has stuck with you?
Brittany: Most of the experiments were performed on late-stage embryos and ammocetes that weren’t much larger, and as a result, a lot of our analyses documented sympathetic progenitors and immature neurons. To get a more mature population of sympathetic neurons for the final sequencing experiment, we actually had to source much larger, older ammocetes directly from the Great Lakes off-season. When they arrived at the lab, I was a bit shocked. They were so much larger than anything I was accustomed to working with, and I wasn’t sure if my tools were even appropriate for the dissections. The long and the short of it: the dissections were fine, but more importantly, the sympathetic trunks of these later-staged ammocetes were visibly discernible under the dissecting microscope. Of course we trusted our data from the late-stage embryos and the younger ammocetes, but I think it’s true that seeing is believing.
And the flipside: any moments of frustration or despair?
Brittany: When it comes to experiments and data, I try to keep a level head and clear perspective. As scientists, we are after the truth, and every clear result (even the ones we didn’t want or expect) gets us closer to the truth. The scientific process really does work, and having trust in that goes a long way on more challenging days.
What’s next for you, Brittany?
Brittany: Ultimately, I would like to be an independent investigator. I’m drawn to comparative embryology as a means to understand how peripheral sensory and autonomic neural systems first arose in vertebrates, and how genetic and environmental changes have driven diversification and adaptation of these systems over time. That’s a bit longer term, since I’ve just crossed the three-year mark as a postdoc, but in the meantime we have a collaboration with the Cai Lab at Caltech that I’ve been very excited about. We are looking to leverage their spatial barcoding technology, seqFISH, to better understand neuronal heterogeneity within the peripheral nervous system. Another endeavor I would like to mention is the work I’ve been doing with the support of the Caltech CTLO (Center for Teaching, Learning, and Outreach). One of my goals is to make hands-on science education more accessible to younger students, and with support from the CTLO and feedback from our local high school students, I have been developing grade-appropriate protocols and resources to introduce topics in embryology and neurobiology.
And Marianne, where will this story take the lab?
Marianne: We are continuing to work on many different neural crest derivatives in lamprey and would like to understand whether the gene regulatory circuits resulting in neural crest differentiation into things like peripheral neurons, craniofacial structures, etc. are conserved to the base of vertebrates. Right now, we are particularly interested in the enteric nervous system and how it has become elaborated.


 (3 votes)
(3 votes)






 (No Ratings Yet)
(No Ratings Yet)