In Development this week (Vol. 144, Issue 14)
Posted by Seema Grewal, on 18 July 2017
Here are the highlights from the current issue of Development:
Computing branching pattern complexity

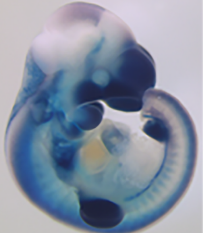
Bile – a fluid that aids digestion – is transported from the liver to the intestine through the bile duct. Bile reaches the bile duct itself via a complex, highly branched structure called the intrahepatic biliary network. This network spreads throughout the liver but how it is patterned is unclear. On p. 2595, Takuya Sakaguchi and colleagues report a novel computational approach to analyse the 3D structure of this network in developing zebrafish. They use a computational algorithm that renders confocal scans of labelled livers into compact representations of the intrahepatic biliary network, which recapitulate endogenous branching patterns and simplify the branched networks into segments amenable to further analysis. Using this computational approach, the authors identify a small molecule inhibitor of Cdk5 that reduces the density of the biliary network, leaving liver size and biliary epithelial cell numbers unchanged. They also experimentally manipulate the downstream Cdk5-Pak1-LimK-Cofilin cascade to increase branching density, and demonstrate a role for this cascade in regulating actin dynamics in biliary epithelial cells. These findings demonstrate the utility of this computational approach to studying branched tissues and highlight the Cdk5-Pak1-LimK-Cofilin cascade as a potential therapeutic target for liver disorders.
No lung development without Sin(3a)

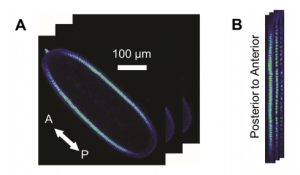
Sin3a is a co-repressor that modulates the transcription of numerous genes by complexing with chromatin remodelling enzymes that modify histones. Here, Barry Stripp and co-workers reveal a key role for Sin3a during lung development in mice (p. 2618). They report that the foregut endoderm-specific deletion of Sin3a leads to failed lung development and the death of neonatal pups due to respiratory failure. Although lung buds form in early mutant embryos, subsequent branching morphogenesis and development fails, with loss of both the lung endoderm and lung epithelium. Loss of Sin3a also disrupts lung mesoderm differentiation, possibly due to aberrant epithelial-mesenchymal interactions. Interestingly, histone H3 acetylation levels show no significant change in Sin3a-deficient epithelial cells, indicating that loss of histone deacetylation activity is unlikely to contribute to the lung phenotype. Instead, lung epithelial progenitor cells in mutant embryos enter a senescence-like state and arrest in G1. This cell cycle arrest is partially mediated by upregulation of the cell cycle inhibitors Cdkn1a and Cdkn2c. Together, these findings reveal that Sin3a plays a crucial role in regulating early lung endoderm progenitor cell fate, via the transcriptional repression of cell cycle inhibitors, to prevent the induction of a senescence-like state. Whether Sin3a plays a similar role in the postnatal lung awaits further investigation.
Set(d1b)ing oocyte gene expression

Mouse primordial germ cells (PGCs) – the precursors of oocytes and sperm – undergo extensive DNA demethylation as they migrate to the genital ridge. In primary oocytes, DNA undergoes re-methylation, and lysine residues on the tails of histone H3 become methylated. This important epigenetic mark is created in mammals by H3K4 methyltransferases, including Setd1a and Setd1b. Setd1a plays no role in oogenesis, but now Andrea Kranz and colleagues report that the loss of Setd1b in mouse oocytes causes female sterility (p. 2606). Although metaphase II-stage oocytes develop in female Setd1bconditional mutants, both their zona pellucida and meiotic spindle are abnormal. Upon fertilisation, extra sperm enters the perivitelline space of the mutant oocytes and the zygotes become stuck at the pronuclear stage. RNA profiling reveals that Setd1b is required for the expression of key oocyte transcription factors and that its inactivation causes twice as many mRNAs to be upregulated as downregulated. Thus, Setd1b likely promotes the expression of transcriptional repressors, possibly Zfp-KRAB factors, which maintain the oocyte-specific expression programme in late development by reducing earlier, unwanted gene expression. These findings reveal a novel role for Setd1b in regulating the oocyte-to-embryo transition, possibly by regulating the late-oocyte gene expression programme.
PLUS…
The status of the human embryo in various religions
 Research into human development involves the use of human embryos and their derivative cells and tissues. How religions view the human embryo depends on beliefs about ensoulment and the inception of personhood, and science can neither prove nor refute the teaching of those religions that consider the zygote to be a human person with an immortal soul. In his Spotlight article, William Neaves discusses some of the dominant themes that have emerged with regard to how different religions view the human embryo, with a focus on the Christian faith as well as Buddhist, Hindu, Jewish and Islamic perspectives.
Research into human development involves the use of human embryos and their derivative cells and tissues. How religions view the human embryo depends on beliefs about ensoulment and the inception of personhood, and science can neither prove nor refute the teaching of those religions that consider the zygote to be a human person with an immortal soul. In his Spotlight article, William Neaves discusses some of the dominant themes that have emerged with regard to how different religions view the human embryo, with a focus on the Christian faith as well as Buddhist, Hindu, Jewish and Islamic perspectives.
Interspecies chimeras for human stem cell research
Interspecies chimeric assays are a valuable tool for investigating the potential of human stem and progenitor cells, as well as their differentiated progeny. In their Spotlight article, discuss the different factors that affect interspecies chimera generation, such as evolutionary distance, developmental timing, and apoptosis of the transplanted cells, and suggests some possible strategies to address them.
A framework for understanding the roles of miRNAs in animal development
 MicroRNAs (miRNAs) contribute to the progressive changes in gene expression that occur during development. In their Review, present a view of miRNAs as a hierarchical and canalized series of gene regulatory networks. In this scheme, only a fraction of embryonic miRNAs act at the top of this hierarchy, with their loss resulting in broad developmental defects, whereas most other miRNAs are expressed with high cellular specificity and play roles at the periphery of development, affecting the features of specialized cells.
MicroRNAs (miRNAs) contribute to the progressive changes in gene expression that occur during development. In their Review, present a view of miRNAs as a hierarchical and canalized series of gene regulatory networks. In this scheme, only a fraction of embryonic miRNAs act at the top of this hierarchy, with their loss resulting in broad developmental defects, whereas most other miRNAs are expressed with high cellular specificity and play roles at the periphery of development, affecting the features of specialized cells.


 (No Ratings Yet)
(No Ratings Yet)
 (1 votes)
(1 votes)
















 (7 votes)
(7 votes)