Blastoid: the backstory of the formation of blastocyst-like structure solely from stem cells.
Posted by Nicolas Rivron, on 27 June 2018
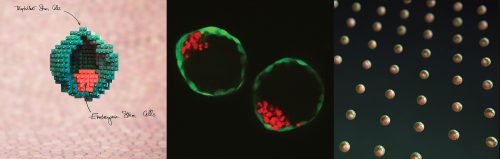
In our recently published paper1, we showed that mouse stem cells self-organize into blastocyst-like structures, that we termed blastoids. Because blastoids can be generated in large numbers, can be finely manipulated, and implant in utero, they are a powerful tool to investigate the principles of pre- and post-implantation development. Here is the backstory of our discovery and why we think it is important for science and medicine.
Nature | doi:10.1038/s41586-018-0051-0
The early mammalian embryo, known as the blastocyst, combines the simple and esthetic design of an outer cyst (the future placenta) englobing an inner cell cluster (the future embryo) with the powerful potential to form the whole organism (both embryonic and extra-embryonic). But blastocysts are also few, small, with a small number of cells (<100), and are difficult to physically and genetically manipulate2. Many of its operating principles therefore remain unknown.
Stem cell-based embryology.
Stem cells have the intrinsic capacity to self-organize in vitro, as shown with post-implantation models known as gastruloids3-5 or with organoids. Here, we created the first version of a pre-implantation blastocyst model by promoting the self-organization of mouse embryonic (ESCs) and trophoblast stem cells (TSCs).
The reductionist approach of forming entities ‘from the bottom up’ allows to modulate and reveal previously unnoticed principles of development. In addition, blastoids have technical advantages as compared to blastocysts. Large number of genetically similar structures can be generated, thus opening possibilities for high-throughput screens and for biochemistry-based assays. Also, sub-populations can be rapidly and efficiently fine-tuned. For instance, the trophoblast and embryonic compartments can be physically and genetically modified independently one from another (e.g. dosing/mixing of different genotypes)1, 2.
Altogether, blastoids are models to study the genetically-encoded principles of self-organization, and to generate novel hypotheses on development, which complement the classical ‘top-down’ approach (e.g. observing genetically modified blastocysts). In the future, blastoids, which comprise the cell types necessary to form the whole organism and which can be transferred in utero, might form a full organism solely from stem cells.
Pulling forces to initiate the project.
As an undergraduate in France, I studied a range of physics and engineering-related topics such as polymer physics and fluid dynamics, which are statistical and modelling sciences. However, what grasped my imagination was the experimental concept of tissue engineering as proposed by Linda Griffith and Robert Langer6. I applied it during my PhD (2006-2010) by studying the self-organization of pre-vascularized engineered tissues (Prof. Clemens van Blitterswijk, University of Twente, The Netherlands). At the end of it, Clemens van Blitterswijk gave me the freedom to work independently, and to hire two PhD students. A great opportunity to develop new ideas, and at an interesting time: the laboratories of Hans Clevers (Hubrecht Institute) and of Yoshiki Sasai (CDB RIKEN) had just shown that stem cells can form organoids. These discoveries perfectly balanced self-organization and developmental biology.
As I wanted to model the embryo, the blastocyst appeared as a lucid choice in light of the available ESCs and TSCs types. The necessity to gain knowledge on blastocysts stem cells led me to ask Niels Geijsen to become a guest at the Hubrecht Institute for developmental biology and stem cell research (Utrecht, The Netherlands). I obtained an additional grant (“Modulating trophectoderm pluripotency and placental development in artificial blastocysts”, ZonMw project number 116005008), hired two great PhD students Erik Vrij (2011) and Javier Frias Aldeguer (2012), and started pipetting ESCs and TSCs, while finalizing the publication of my PhDs’ papers (2012)7,8. Erik focused on developing high-content imaging to quantify the phenotypes, while Javier took a deep dive into the relatively unexplored biology of TSCs.
It took us years of intense, stressful and risky work, and we had to run less uncertain projects to maintain a reasonable output, but the team progressively gained expertise and developed efficient, robust experimental pipelines.
How did it come to work?
Self-organization relies on setting up the right boundary conditions to trigger the process. Once initiated, the stem cells remember where they come from, and unleash their intrinsic potential. Two elements were key in creating these initial conditions, which also apply to other self-organizing biological systems. First, a fine control over the confinement of minute, precise number and ratios of ESCs and TSCs. We achieved this using a hydrogel microwell array that I designed and fabricated during my PhD8. Second, the possibility to screen for combinations of molecules to trigger the process. We did this by combining transcriptomic databases with the knowledge on signaling pathways previously found by many blastocyst and stem cells labs. The list is long here but we were definitely standing on the shoulders of Janet Rossant, Jennifer Nichols, Austin Smith.
Our approach suggested new mechanisms. For instance, many Wnt ligands are known to be produced by the blastocyst cells (e.g. Wnt7b and Wnt6 by the trophoblasts9) but their functions are not known (knock-out mice are not informative10, possibly due to the plasticity and redundancy of pathways). I clearly remember when stimulating the cells with Wnt activators (Wnt3a, CHIR99021) and looking at the plates 48 hours later. This triggered the cavitation of TSCs, resulting in gorgeous trophectoderm-like cysts. We thought “phenotypically, it is becoming really good and efficient. We might get somewhere, but are we looking at something really occurring in the trophectoderm?” At the moment, no-one knows but blastoids allow to generate such hypotheses that couldn’t be tested until now.
Once the initial conditions are gathered, including a cocktail of six molecules, the stem cells spontaneously organize within 65 hours. The process is rapid and efficient: about 70% of the microwells that include an adequate number of stem cells form a blastoid (see our definition of a blastoid1).
Making discoveries at the single cell level.
In the meantime, the Hubrecht Institute changed director. Hans Clevers stepped down to focus on national-level activities, and Alexander van Oudenaarden came back from MIT to take the job. He set up a large lab focusing on single cell technologies, which attracted our attention as a powerful way to reveal the gene expression patterns underlying the self-organization of blastoids.
We undertook a series of experiments with Jean-Charles Boisset, postdoc in Alexander’s lab. The initial bulk sequencing run comparing blastoids and blastocysts was probably the second convincing moments that made us feel that we were going in the right direction. With his natural phlegm, Jean-Charles pushed the button on his computer to generate the distance map and simply said “it seems to work”, as a heat-map came out on his screen. The transcriptome of blastoid cells was shifting toward the one of a blastocyst, and we had the green light to depict the phenomena at the single cell level. By physically decoupling the compartments (ESCs or TSCs alone versus blastoid cells) and comparing the single cells with the ones from blastocysts, we pinpointed at changes and at the role of the communication between the TSCs and ESCs.
The ESCs clearly played an inductive role: maintaining trophoblast proliferation, inducing morphogenesis, gate-keeping the progression of trophoblast differentiation, and altogether preserving the potential for the trophectoderm to implant in utero. We established a long list of embryonic inductions that guide trophectoderm development (e.g. metabolic, Jak/STAT, Wnt, SMAD, and Hippo pathways), all of which were interesting mechanisms probably contributing to the formation and implantation of the blastocyst.
Reviewing, forever.
It took us two years to convince the reviewers that the blastoid system was modeling relevant aspects of blastocyst development. We were asked, among many other experiments, to obtain phenotypes upon generation of KO within ESCs and within TSCs. We replaced the ESCs by other cell types to prove the specificity of the embryonic inductions, by the factor that they secrete as well (BMP4, Nodal), and ran high-throughput phenotypic screens to quantify the morphogenetic and functional impact of embryonic inducers.
The in-utero transfer assay was probably crucial to tip the balance as, for the first time, stem cells alone formed a full entity that could be transferred and tested in utero. We did not form a bona fide embryo in utero but blastoids implanted and induced the expression of Aldh3a1 in the deciduae, which is thought to be a specific response to blastocyst implantation (as compared to polymer beads)11. Blastoid cells proliferated, elongated and generated multiple relevant cell types, including giant trophoblasts that hooked up with the mother’s blood system.
Finally, the paper was released in May 2018, and gained attention from the media. I interviewed for the BBC, BBC World News (live!), CNN or Fortune, and more than 100 press articles were released worldwide. We managed to restrain the unfounded fantasy for unethical human reproduction approaches, and to focus on the opportunities to research in the lab the fundamental principles of development or the minor flaws that can occur at the start of pregnancy. These flaws can prevent the conceptus to implant or can contribute to sub-optimal pregnancies (e.g. sub-optimal placenta development) affecting the appearance of chronic diseases during adult life. The biology community also efficiently relayed realistic and positive potential impacts. As free-electrons in stem cell biology and embryology, it has been gratifying to see the positive and encouraging reactions of valued scientists12-14. A Tweet worth years of research15.
Imagining the future of blastoids.
Along the way, curiosity led me to discuss with clinicians including human geneticists, epidemiologists, or IVF clinicians. We started to think of realistic possibilities to use blastoids to research in the lab the problems of infertility, contraception, or early pregnancy failure12. These are extensive societal problems: On one side, humans, which are poorly efficient at procreating, currently delay more and more their pregnancy, which leads to a drop of fertility16. On the other side, family planning and contraception remains a major global health problem as depicted by WHO17 and the Bill & Melinda Gates foundation18.
Overall, women must be able to better plan their pregnancy without decreasing their chance of having a child. Family planning is a huge lever to secure women’s autonomy and well-being, while supporting the health and development of communities. There is a long way in front of us but we are thrilled to see that we might be able to reveal new principles in embryology and, along with clinicians, tackle global health problems.
We are currently recruiting Postdocs and PhD students. Feel free to contact me for more information. www.nicolasrivron.org
[1] Rivron NC [corresponding author], Frias-Aldeguer J, Vrij EJ, Boisset JC, Korving J, Vivié J, Truckenmüller RK, van Oudenaarden A, van Blitterswijk CA †, Geijsen N † [† equal contribution]. Blastocyst-like structures generated solely from stem cells. Nature. 2018. 557, pages106–111 (2018). doi:10.1038/s41586-018-0051-0.
[2] Rivron NC. Formation of blastoids from mouse embryonic and trophoblast stem cells. Protocol Exchange (2018) doi:10.1038/protex.2018.051
[3] van den Brink SC, Baillie-Johnson P, Balayo T, Hadjantonakis AK, Nowotschin S, Turner DA, Martinez Arias A. Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Development. 2014 Nov;141(22):4231-42. doi: 10.1242/dev.113001.
[4] Harrison SE, Sozen B, Christodoulou N, Kyprianou C, Zernicka-Goetz M. Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science. 2017 Apr 14;356(6334). pii: eaal1810. doi: 10.1126/science.aal1810.
[5] Warmflash A, Sorre B, Etoc F, Siggia ED, Brivanlou AH. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Methods. 2014 Aug;11(8):847-54. doi: 10.1038/nmeth.3016.
[6] http://news.mit.edu/2012/engineering-health-tissue-engineering-growing-organs-1214
[7] Rivron NC, Raiss CC, Liu J, Nandakumar A, Sticht C, Gretz N, Truckenmüller R, Rouwkema J, van Blitterswijk CA. Sonic Hedgehog-activated engineered blood vessels enhance bone tissue formation. Proc Natl Acad Sci U S A. 2012 Mar 20;109(12):4413-8. doi: 10.1073/pnas.1117627109.
[8] Rivron NC, Vrij EJ, Rouwkema J, Le Gac S, van den Berg A, Truckenmüller RK, van Blitterswijk CA. Tissue deformation spatially modulates VEGF signaling and angiogenesis. Proc Natl Acad Sci U S A. 2012 May 1;109(18):6886-91. doi: 10.1073/pnas.1201626109.
[9] Kemp C, Willems E, Abdo S, Lambiv L, Leyns L. Expression of all Wnt genes and their secreted antagonists during mouse blastocyst and postimplantation development. Dev Dyn. 2005 Jul;233(3):1064-75.
[10] van Amerongen R, Berns A. Knockout mouse models to study Wnt signal transduction. Trends Genet. 2006 Dec;22(12):678-89.
[11] McConaha, M. E., Eckstrum, K., An, J., Steinle, J. J. & Bany, B. M. Microarray assessment of the influence of the conceptus on gene expression in the mouse uterus during decidualization. Reproduction 141, 511–527 (2011).
[12] Rossant J, Tam PPL. Exploring early human embryo development. Science. 2018 Jun 8;360(6393):1075-1076. doi: 10.1126/science.aas9302.
[13] https://f1000.com/prime/733152526#eval793546032
[15] https://twitter.com/AMartinezArias/status/991729701130563584
[16] https://www.nytimes.com/2018/05/17/us/fertility-rate-decline-united-states.html
[17] http://www.who.int/news-room/fact-sheets/detail/family-planning-contraception
[18] https://www.gatesfoundation.org/What-We-Do/Global-Development/Family-Planning


 (4 votes)
(4 votes)