Wellcome Trust Independent Senior Research Fellow at Warwick Medical School
Posted by Karuna Sampath, on 27 January 2016
Closing Date: 15 March 2021
http://www.jobs.ac.uk/job/AUC078/wellcome-trust-independent-senior-research-fellow-75344-016/
The University of Warwick has initiated a Wellcome-Trust funded research programme in “Quantitative Biomedicine” to bridge physical / mathematical sciences and biomedicine. The programme is of a cross-campus nature with strong participation from the Division of Biomedical Cell Biology and the Warwick Systems Biology Centre.
We are seeking early career researchers who are no more than 4 years from obtaining a Doctoral degree with the potential to lead a strong independent research programme. The candidates will be selected based on their track record in innovative research and on the strength of the research proposal. Importantly, the proposals should make clear how the project benefits from quantitative methodologies. The proposed research programme should bring together physical and mathematical sciences and biomedical sciences (including cell and developmental biology, neurobiology, immunology, microbiology and infection). Identified candidates will be provided salary, laboratory space, running costs and part-time technical support for three years. The successful candidates will be expected to win externally funded fellowships within the contract period and will be mentored in preparing such applications.
Interested candidates should submit their CV, a two-page research proposal, and the names and addresses of three referees who are able to comment on the candidates past research work as well as the readiness of the candidate to embark on an independent career in research.


 (No Ratings Yet)
(No Ratings Yet)
 (1 votes)
(1 votes)
 As you may know, The Company of Biologists runs a
As you may know, The Company of Biologists runs a 

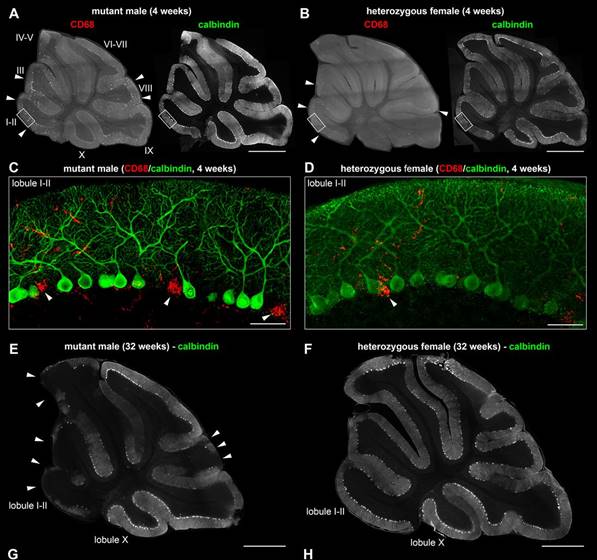 Mutations in SLC9A6 are responsible for X-linked Christianson syndrome, a neurodevelopmental disease. Sikora and colleagues demonstrate that female mice heterozygous for a Slc9a6 knockout present mosaic neuropathology and similar but milder behavioural traits to those of affected males. Read the paper
Mutations in SLC9A6 are responsible for X-linked Christianson syndrome, a neurodevelopmental disease. Sikora and colleagues demonstrate that female mice heterozygous for a Slc9a6 knockout present mosaic neuropathology and similar but milder behavioural traits to those of affected males. Read the paper 
 Sp5 has been identified as an effector of both Wnt/β-catenin and leukemia inhibitory factor (LIF)/Stat3 pluripotency signaling, and its forced expression produces effects of both pathways in mESC pluripotency. Furthermore, Sp5 can convert mouse epiblast stem cells into mESCs. Read the paper
Sp5 has been identified as an effector of both Wnt/β-catenin and leukemia inhibitory factor (LIF)/Stat3 pluripotency signaling, and its forced expression produces effects of both pathways in mESC pluripotency. Furthermore, Sp5 can convert mouse epiblast stem cells into mESCs. Read the paper 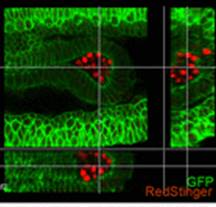 In this paper, Parès and Ricardo describe how fibroblast growth factor (FGF) signaling modulates zygotic E-cadherin distribution to maintain posterior midgut epithelial 3D architecture, impacting on primordial germ cell motility during the early embryonic development of Drosophila. Read the paper
In this paper, Parès and Ricardo describe how fibroblast growth factor (FGF) signaling modulates zygotic E-cadherin distribution to maintain posterior midgut epithelial 3D architecture, impacting on primordial germ cell motility during the early embryonic development of Drosophila. Read the paper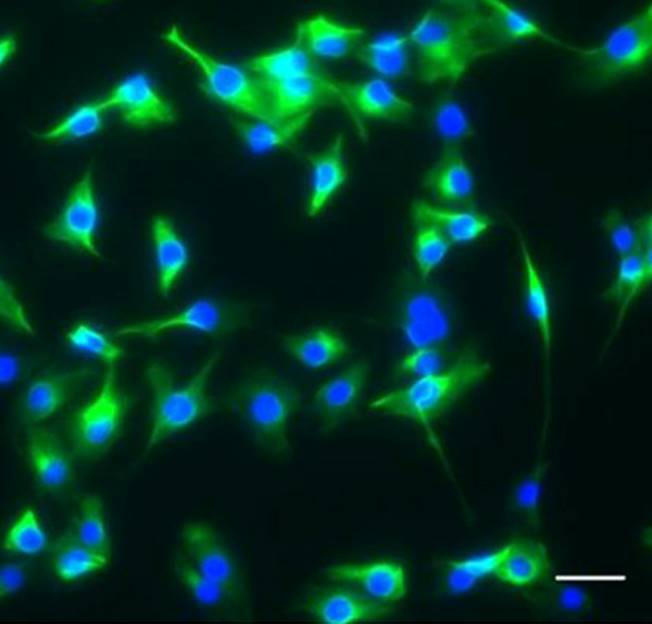 Wei and colleagues show that miR-195 and miR-497 target lgf1r, lnsr, Ccnd2 and Ccne1 and inhibit proliferation in C2C12 cells. They also show that these microRNAs are negatively regulated by nuclear factor κB, illustrating an important signalling pathway in myogenesis. Read the paper
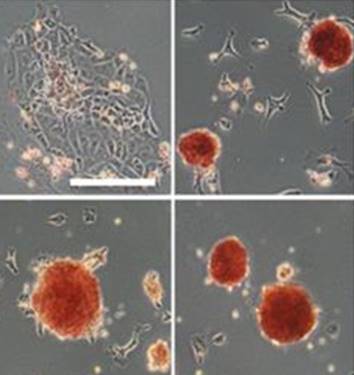
Wei and colleagues show that miR-195 and miR-497 target lgf1r, lnsr, Ccnd2 and Ccne1 and inhibit proliferation in C2C12 cells. They also show that these microRNAs are negatively regulated by nuclear factor κB, illustrating an important signalling pathway in myogenesis. Read the paper 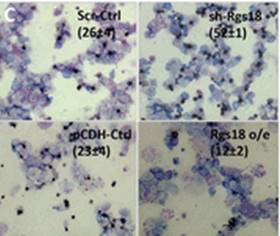 Sengupta and colleagues describe how Rgs18, a GTPase-activating protein, and its transcriptional repressor Gfi1b reciprocally regulate the lineage segregation between the megakaryocytic and the erythroid lineage through the downstream effects on the antagonistic transcription factors Fli1 and Klf1. Read the paper
Sengupta and colleagues describe how Rgs18, a GTPase-activating protein, and its transcriptional repressor Gfi1b reciprocally regulate the lineage segregation between the megakaryocytic and the erythroid lineage through the downstream effects on the antagonistic transcription factors Fli1 and Klf1. Read the paper 