A new look at the (microscopic) world- 350 years since Hooke’s landmark book
Posted by Catarina Vicente, on 9 November 2015
‘These pores, or cells, were not very deep, but consisted of a great many little Boxes … [they] were indeed the first microscopical pores I ever saw, and perhaps, that were ever seen’
Last month I was fortunate enough to attend the conference ‘Cell: from Robert Hooke to Cell Therapy- a 350 year journey’, which took place at the Royal Society in London. The focus of the meeting was new advances in cell therapies, particularly in the field of stem cells, and featured a stellar line-up of speakers. However, it was the first talk, given by historian Felicity Henderson, that really grabbed my attention. It highlighted an important anniversary that we are celebrating this year- the 350 years since Robert Hooke published his book Micrographia.
So why was Micrographia such an important book, and why should we, scientists, care about it? Well, it was quite simply the first illustrated book of microscopy. Hooke was not the first person to use a microscope. His Micrographia was published in 1665, but the microscope had already been around for a few years (since 1648 to be precise). However, Hooke was really the first person to make what you could see under the microscope accessible to anyone, even if you were not a scientist. So how did it fall onto him to write the first microscopy book?
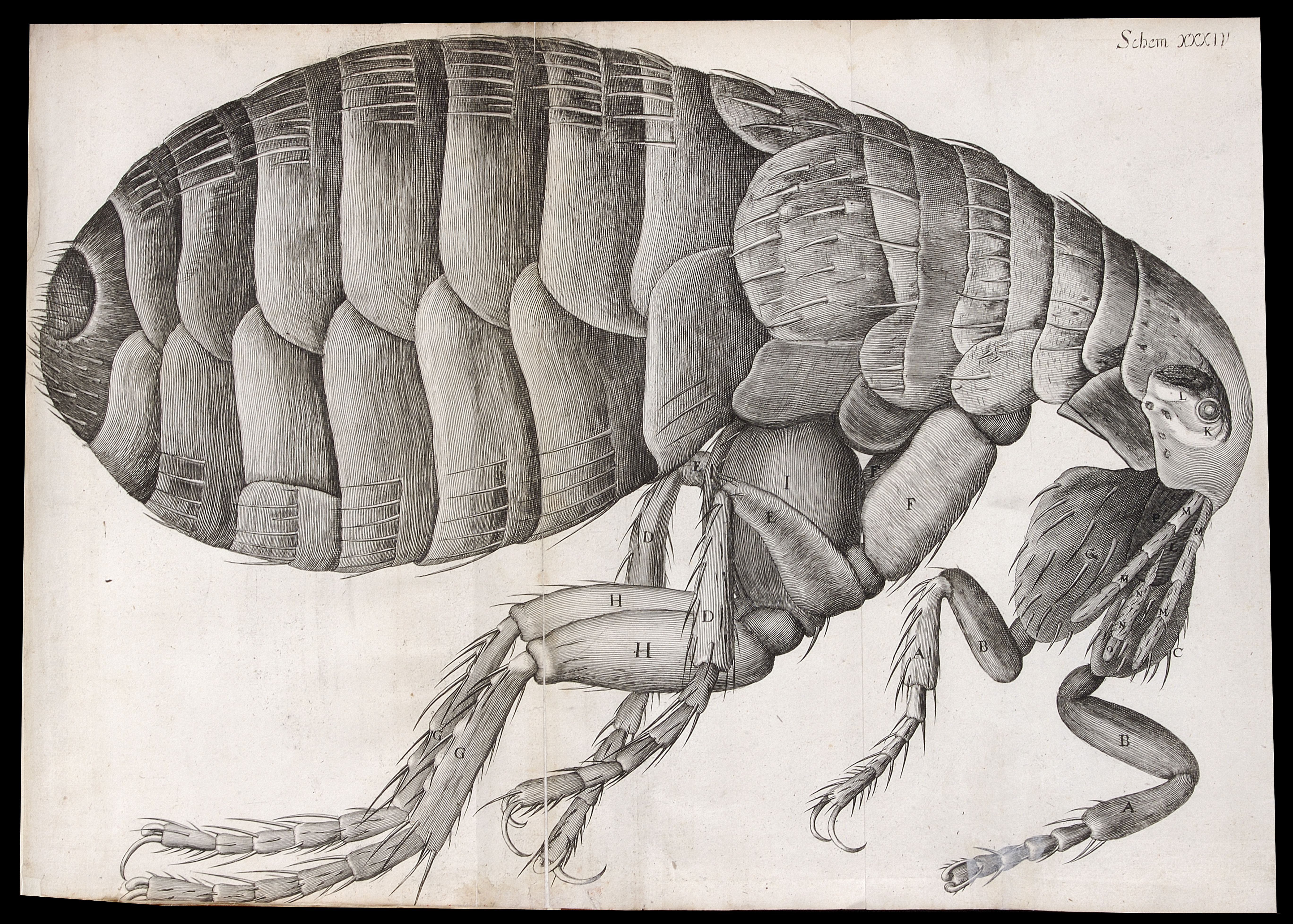
Microscopic view of a flea, Micrographia. Reproduced with permission by The Royal Society ©The Royal Society
Hooke was employed by the Royal Society, and in some ways he can be considered the first professional research scientist in the UK since, unlike other wealthy natural philosophers who had private means, Hooke was actually paid a salary by the Society. Within the Society he was responsible for a variety of activities, including preparing exciting practical experiments for the entertainment and interest of the other fellows. The Royal Society was, in Hooke’s days, a newly founded institution, created in 1660 under the auspices of King Charles II. Charles II was very interested in science, and particularly enjoyed the images of microscopic creatures that Sir Christopher Wren (then the president of the Society) had given to him as a gift (and which he displayed alongside other works of art in his residence). In fact, Charles II liked them so much that he asked for more. In order to ‘win favour’ with their royal patron, the Society decided to publish a book (one of its first publications as an institution) showcasing the exciting microscopic science that was starting to be explored. Sir Cristopher Wren was too busy with other projects (designing St Paul’s Cathedral, for example?) so he asked Hooke to do it instead. The result of his efforts was a beautiful illustrated (and rather expensive) volume, worthy of a king! The project was a huge success with the public. Samuel Pepys, of the famous diary, said that it was so fascinating that he apparently stayed up all night reading it!
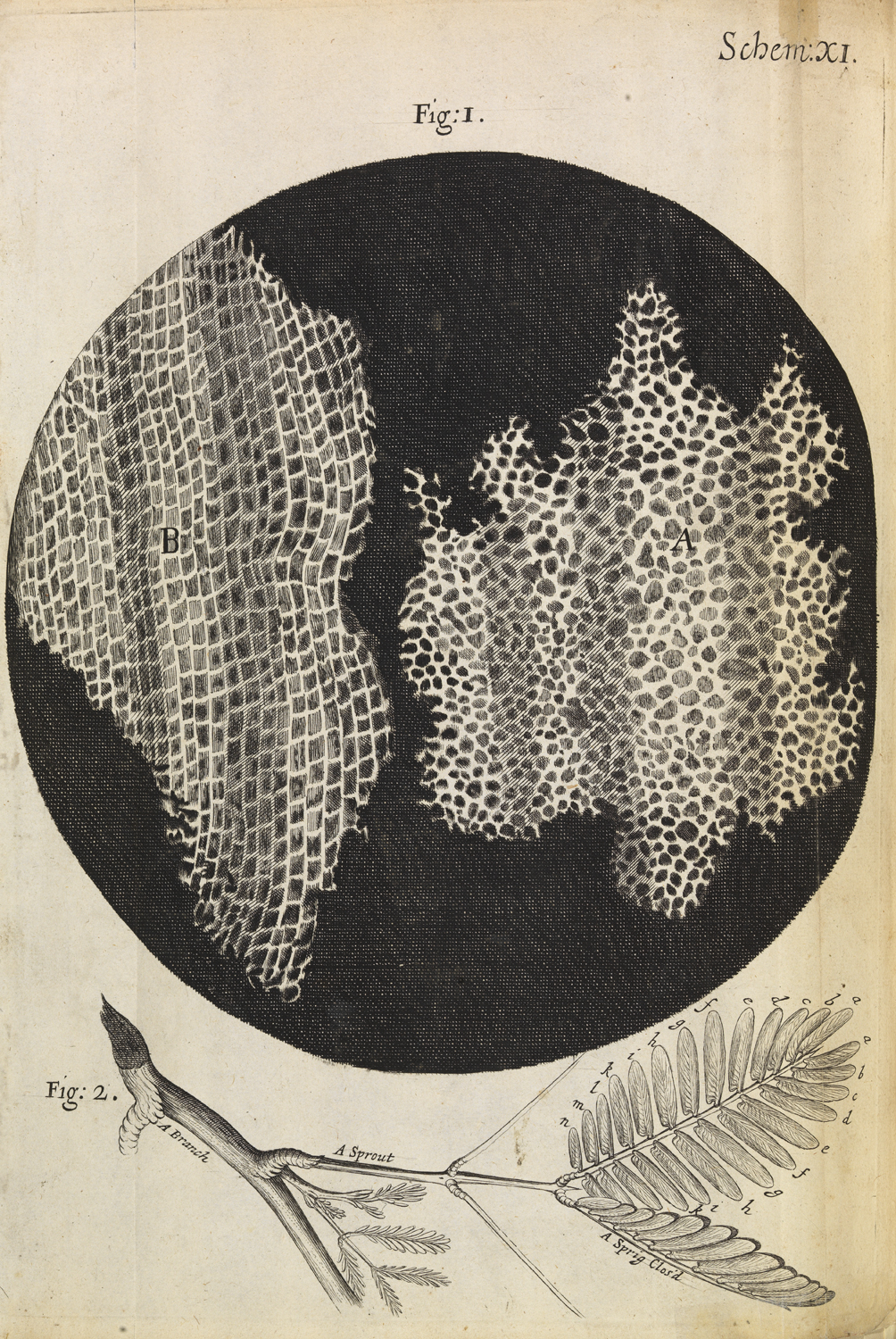
So why was Micrographia so popular? The most obvious feature is its beautiful (and numerous) illustrations. At a time when there were only a few microscopes (and no google), the book allowed the common man to see what was normally unseen. Hooke made all the illustrations himself. Before becoming a scientist he actually worked for some time in an artists’ workshop (although eventually left because the constant smell of paint made him sick!), so he had some formal training. However, the subjects of his illustrations were probably as important as their artistic qualities. One of the things I was most surprised to hear about in this talk was how sensitive Hooke was to what would grab the public’s interest. He may have been a 17th century scientist, but he had very 21st century ideas about what makes good science communication! He was aware that a way to make the work interesting to the public would be by focusing on everyday objects and critters. The very first image in the book, for example, features a needle, a printed full stop and a razor blade. One of the most popular images in the book (both then and today) is a big fold-out image of a flea, ‘the size of a cat’, and when Hooke wanted to show an image of a louse, he showed it holding on to a human hair (which is where most people at the time would come across it). Hook also understood the importance of using simple language that the public could follow. Micrographia was the book that coined the word ‘cell’. Hooke used it when describing a very thin slice of cork. In this very thin slice you could see perforated holes. Instead of calling them ‘globules’ (a much more common word to describe microscopy structures at the time), Hooke called these holes ‘cells’, because they resembled honeycomb cells, something that the public was much more familiar with.
Microscopic view of cells in a sliver of cork, Micrographia. Reproduced with permission by The Royal Society ©The Royal Society
The way Hooke discussed the slices of cork is also a great example of something else that interested him. Explaining that the ability to see the microscopic world was not just a curiosity-it could help us understand how cells and organisms work. This was part of a new trend in the 17th century, a ‘new philosophy’, that focused on understanding how the world works in a mechanical way. Take the slice of cork. Hooke argued that this porous microscopic structure explained the properties of cork- how it was light and compressible. The beginnings of material science! Similar structures could also tell us about relatedness and natural history. Hooke noticed that charcoal and wood looked very similar under the microscope, displaying similar pores or ‘cells’, and this supported his theory that fossils are the remains of previous existing organisms. And, as if he was under the pressure of current funding trends, he hypothesised that some of these microscopic structures could be exploited or adapted into practical applications. He was intrigued, for example, by the sharp needles that he could observe in the leaves of stinging nettles. He could see that they were hollow and after some experimentation (by stinging himself repeatedly!) he concluded that they worked as syringes and hypothesised that similar structures might be used for medicinal purposes. Finally, he didn’t want the public to think that microscopy was some magic trick, and provided detailed instructions on how he prepared his specimens (in case you are wondering, ants move too much to look at them under the microscope, but dumping them in brandy for an hour does just the trick!).
Hooke was clearly a man ahead of his time, not only as a scientist but also as a communicator. Interestingly enough, and not unlike many of today’s communication efforts, his beautiful illustrations, useful metaphors and discussions of practical applications did not convince everyone. After all, what could be the use of spending your time looking at lice at such detail? It seems that the fight to persuade the public of the importance of science is several centuries long!
If you are interested in having a look at Micrographia yourself, head out to The Royal Society in London, where until the 17th of December you can see a small exhibition on the history of microscopy, including a first edition of this book. You can also find out more information about Hooke at Felicity’s blog Robert Hooke’s London.
A copy of Micrographia at The Royal Society.



 (4 votes)
(4 votes) (No Ratings Yet)
(No Ratings Yet)








 Strigolactones (SLs), first identified for their role in parasitic and symbiotic interactions in the rhizosphere, constitute the most recently discovered group of plant hormones. In their poster article, Catherine Rameau and colleagues summarize current understanding of the SL pathway and discuss how this pathway regulates plant development. See the Development at a Glance article on p.
Strigolactones (SLs), first identified for their role in parasitic and symbiotic interactions in the rhizosphere, constitute the most recently discovered group of plant hormones. In their poster article, Catherine Rameau and colleagues summarize current understanding of the SL pathway and discuss how this pathway regulates plant development. See the Development at a Glance article on p.  The sense of taste, or gustation, is mediated by taste buds, which are housed in specialized taste papillae found in a stereotyped pattern on the surface of the tongue. Here, Linda Barlow reviews how the pattern of taste buds is established in embryos and discusses the cellular and molecular mechanisms governing taste cell turnover. See the Review article on p.
The sense of taste, or gustation, is mediated by taste buds, which are housed in specialized taste papillae found in a stereotyped pattern on the surface of the tongue. Here, Linda Barlow reviews how the pattern of taste buds is established in embryos and discusses the cellular and molecular mechanisms governing taste cell turnover. See the Review article on p.