In Development this week (Vol. 139, Issue 4)
Posted by Seema Grewal, on 24 January 2012
Here are the highlights from the current issue of Development:
Neuronal cell fate: windows of opportunity
It is becoming increasingly evident that both vertebrate and invertebrate neural progenitor cells exhibit programmed temporal changes in their competence to generate specific neuronal cell types, but how is this competency controlled? Two articles in this issue address this question by studying Drosophila neuroblast lineage progression.

On p. 657, Michael Cleary and co-workers investigate the role of Polycomb repressor complexes (PRCs) in regulating neuroblast competence. In the Drosophila neuroblast 7-1 (NB7-1) lineage, the transcription factor Kruppel (Kr) specifies the third-born U3 motoneuron, but competence to generate U3 cells is limited to early divisions and is gradually lost when the neuroblasts transition to making interneurons. The researchers show that PRC loss of function extends the ability of Kr to induce U3 fate, whereas PRC gain of function causes precocious loss of competence to make motoneurons. The analysis of other neuroblast lineages that undergo a motoneuron-to-interneuron production transition demonstrates that PRCs also act to restrict motoneuron competence in these lineages. The researchers thus propose a model in which PRCs act to set up motoneuron-specific windows of competence in various neuroblasts that transition from motoneuron-to-interneuron production.
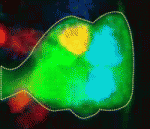
On p. 678, Stefan Thor and colleagues focus on the Drosophila embryonic neuroblast NB5-6T lineage to investigate how cell proliferation and cell fate specification are integrated during development. NB5-6T neuroblasts give rise to a lineage of 20 cells, including a differentiated set of neurons that are born at the very end of the lineage and that express Apterous: the Ap neurons. The authors identify two independent factors, Prospero and Notch, that act in concert to control the proliferation of NB5-6T daughter cells as the lineage progresses temporally; Prospero controls daughter cell proliferation in the early lineage and Notch activity then limits daughter cell proliferation in the late lineage, when Ap neurons are generated directly from neuroblasts, resulting in a programmed differentiation switch. Thus, the authors conclude, the control of neuronal daughter cell proliferation is integrated with temporal progression to ensure that the correct numbers of each unique cell type are generated.
Bypassing auxin signalling

Plant development is regulated by a number of mobile factors. The Arabidopsis BYPASS1 (BPS1) gene was previously shown to control shoot and root development by preventing formation of a mobile compound, but how this compound functions and whether it modulates other signalling pathways is unclear. Now, Leslie Sieburth and colleagues show that Arabidopsis BPS1, as well as two related genes, BPS2 and BPS3, control the production of a mobile factor, the bps signal, which regulates patterning and growth in parallel with auxin signalling (p. 805). By analysing single, double and triple mutants, the researchers show that all three BPS genes control bps signal synthesis. Importantly, bps triple mutants display severe embryogenesis defects, including disruptions to vascular, root and shoot stem cell populations. Finally, bps triple mutants exhibit normal auxin-induced gene expression and localisation of the PIN1 auxin transporter, suggesting that the bps signal functions in an auxin-independent manner. Although the nature of the bps signal remains unknown, these studies identify a novel pathway that regulates multiple stages of plant patterning and growth.
ABC of germline development

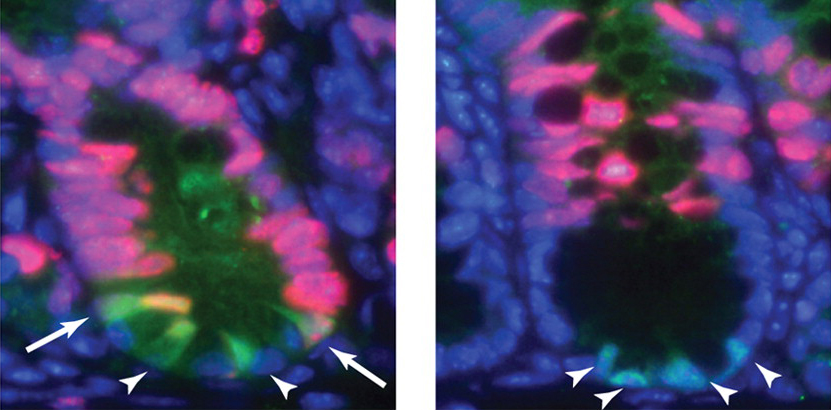
Plasma membrane ABC transporters serve dual functions in the cell: they export toxins to protect against damage and morphogens to mediate communication. It is thought that the activity of ABC transporters in embryos and stem cells should be high, so that mutagens are efficiently removed. Here, Joseph Campanale and Amro Hamdoun (p. 783) report the surprising finding that ABC transporter activity is reduced in germline precursors, the small micromeres, of the sea urchin embryo. This reduction in efflux pump activity can likely be ascribed to an increase in the rate of endocytosis specifically in the micromeres. What are the functional consequences of manipulating ABC transporter activity? The authors take a first step towards understanding this, showing that ABC transporter inhibition disrupts migration of the small micromeres at later stages of embryogenesis. While there is still much to be understood about the regulation and role of these plasma membrane pumps, this study provides evidence for the developmental importance of controlling their surface expression and activity.
Glutamate keeps hair follicles in touch
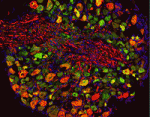
The hairs of our skin are mechanoreceptive: displacement of the hair is detected via sensory afferents in the hair follicle piloneural collar. In this complex structure, neurons, Schwann cells and keratinocytes are closely apposed, and interactions between these three cell types may influence differentiation and function of the piloneural collar. Here, David Owens and colleagues demonstrate that glutamate, which is known to mediate communication between neurons and Schwann cells in the central nervous system, has analogous activities in the periphery (p. 740). Signalling between VGLUT2-expressing neurons and NMDA receptor-expressing Schwann cells directs both formation and maintenance of the piloneural collar in mice. In conditional VGLUT2 mutants, the Schwann cells are disorganised and overall collar structure is severely disrupted. Moreover, treating the skin of adult mice with an NMDA receptor antagonist impairs touch-evoked responses, demonstrating defects in piloneural collar activity. Thus, continuous glutamate signalling between neurons and Schwann cells in the piloneural collar of the skin is essential for the integrity and function of this elaborate mechanosensory structure.
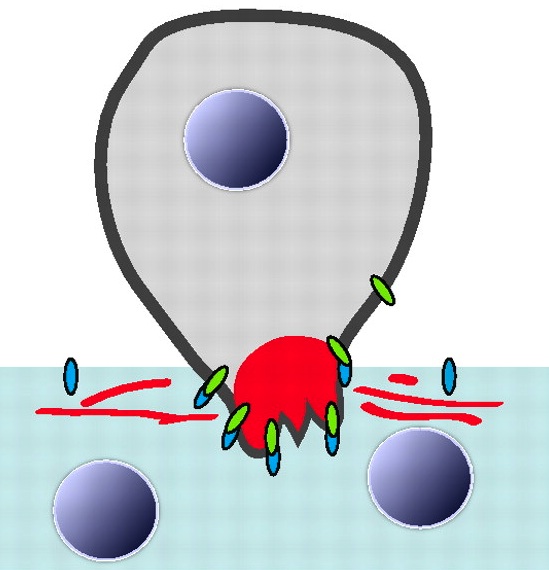
FGF signalling: keeping migrating cells on track
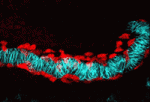
In Drosophila embryos, the longitudinal muscle cells surrounding the gut – the caudal visceral mesoderm (CVM) – arise in the posterior mesoderm and migrate anteriorly to reach their destination where they differentiate. Although this represents the longest cell migration event of Drosophila embryogenesis, the signals directing it are poorly understood. On p. 699, Angelike Stathopoulos and colleagues identify the FGF ligands Pyramus and Thisbe as crucial guidance cues for the CVM, signalling via the Heartless receptor to promote proper migration. The researchers use detailed live imaging and cell tracking analyses to describe wild-type migration, and analyse the consequences of disrupting FGF signalling, revealing defects in migration speed, directionality and cell survival. Intriguingly, by manipulating both the levels and location of ligand expression, they provide evidence for synergistic effects of Pyramus and Thisbe, although the mechanistic basis of such synergism remains to be investigated. Together, these data establish a new system for studying collective cell migration, and suggest additional complexities in FGF ligand-receptor interactions and signalling.
Plus…
Patterning embryos with oscillations: structure, function and dynamics of the vertebrate segmentation clock
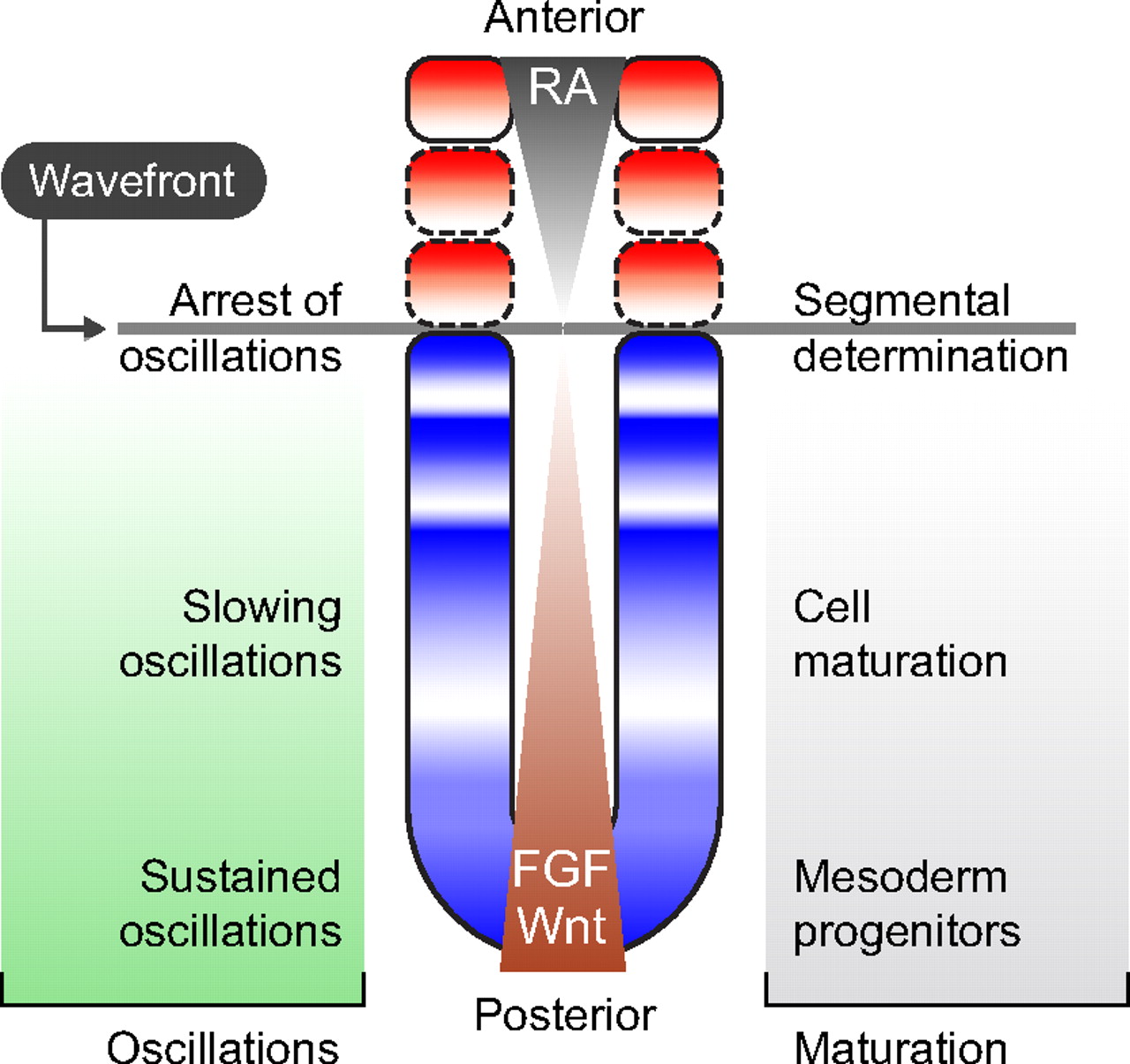
The segmentation clock is an oscillating genetic network thought to govern the rhythmic and sequential subdivision of the elongating body axis of the vertebrate embryo into somites. Recent work, reviewed here by Oates et al, has provided evidence for how the period of the segmentation clock is regulated and how this affects the anatomy of the embryo. See the Review article on p. 625
Myoblast fusion: lessons from flies and mice
The fusion of myoblasts into multinucleate syncytia plays a fundamental role in muscle development and function. Here, Abmayr and Pavlath review the molecular events that drive myoblast fusion in the Drosophila embryo, in developing and regenerating mouse muscle, and in cultured muscle cells. See the Review article on p. 641


 (1 votes)
(1 votes) (No Ratings Yet)
(No Ratings Yet)

 Conference season is about to kick off, so here are a few registration dates for various meetings you might want to attend. If you know of any others, please leave a comment below. And of course keep checking the
Conference season is about to kick off, so here are a few registration dates for various meetings you might want to attend. If you know of any others, please leave a comment below. And of course keep checking the 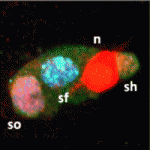



 Somites, confocal, epigenetics, germline, stem cell… BINGO!
Somites, confocal, epigenetics, germline, stem cell… BINGO!