The people behind the papers – Qiongxuan Lu, Yuan Gao and Bo Dong
Posted by the Node Interviews, on 21 June 2021
This interview, the 92nd in our series, was published in Development last year.
In many animal embryos, the tail bends ventrally as it grows, but the underlying mechanisms driving this multi-tissue deformation have been difficult to study. A new paper in Development uses the simple chordate Ciona as a model to study this widely conserved process. To find out more about the story, we met the paper’s two first authors, Qiongxuan Lu and Yuan Gao, and their supervisor Bo Dong, Professor at the Ocean University of China in Qingdao, China.

Bo, can you give us your scientific biography and the questions your lab is trying to answer?
BD: I got my PhD from the Institute of Oceanology, Chinese Academy of Sciences (IOCAS), and then worked as a postdoc in the Sars International Centre for Marine Molecular Biology in the University of Bergen in Norway. After that, I went to RIKEN Centre for Developmental Biology (CDB) in Kobe, Japan, and worked on Drosophila tracheal tube geometry control. In 2014, I came back to the Ocean University of China (OUC) in Qingdao and established my own laboratory working on organ morphogenesis and evolution. My laboratory is principally interested in uncovering the cellular, mechanical and biochemical signalling networks that interact to drive the diverse morphogenetic processes during organ formation and tissue regression using marine ascidians and flies as models.
Qiongxuan and Yuan – how did you come to work in Bo’s lab and what drives your research today?
QL: I first met Bo in 2014 when he gave a lecture related to Ciona notochord tubulogenesis in 2014. From this lecture I was attracted to the field of morphogenesis, and the long-lasting question of how functional shape is generated. I then joined Bo’s lab two years later to investigate the mechanical role of the notochord in chordate embryogenesis. It was really memorable when I took a time-lapse movie on a Ciona embryo without a chorion from zygote to tailbud stage. Indeed, from this movie, we noticed and were curious about the phenomenon of the tail always bending ventrally after the initial tailbud stage, which led us to the story you see in the paper.
YG: Biomechanics has been recognized as the most promising direction of theoretical and applied mechanics. In the Institute of Biomechanics and Medical Engineering (IBME) in Tsinghua University, we focus not only on scientific mechanics problems in crucial biological problems at different length scales, but also emphasize the clinical issues of major diseases. As a PhD student majoring in biomechanics, I am particularly interested in how mechanical forces tune morphogenesis during development, and my PhD project is to develop physical/mechanical models to elucidate these underlying mechanisms.
The mechanisms behind embryonic tail bending in Ciona are so attractive. Thanks to the meeting of Prof. Bo Dong and my supervisor Prof. Xi-Qiao Feng, I was lucky enough to join in this project. Qiongxuan had performed a lot of experiments and obtained interesting and effective results. Based on this, I developed a physical model to further understand the mechanical role of each tissue during the tail bending process in Ciona embryos.
What is the current position of developmental biology and evo-devo research in China?
BD: Currently there is a pretty large developmental biology community in China. We have our own society and hold annual meetings. There are several hundred research groups working on developmental biology-related studies using either classical model animals such as zebrafish, Drosophila and Caenorhabditis elegans, or non-standard model organisms such as ascidian, amphioxus, ciliates and lamprey. Most aspects of developmental biology research – such as organogenesis, pattern formation, physiological metabolism, and regeneration – are categorised as basic research, so the main source of funding is the Natural Science Foundation of China. The evo-devo field is relatively smaller, but the increase in genomic data, new gene editing methods and the fast development of imaging techniques provides us with the opportunity to do evo-devo research in evolutionarily important animals.
Before your work, what was our understanding of how embryonic tail bending was controlled?
QL, YG, BD: Embryonic tail bending is an evolutionarily-conserved morphogenetic process in early embryogenesis for most invertebrates and vertebrates. This large-scale morphogenetic event has been long known about, but the underlying mechanisms have not been investigated. A possible reason is tissue-bending and tissue-folding at the embryo scale is difficult to study because of the anatomical complexity of many model animals. Before our publication, it was thought that embryonic tail bending is a passive process achieved by the physical barrier of the chorion that confines the tail, bending it during elongation.
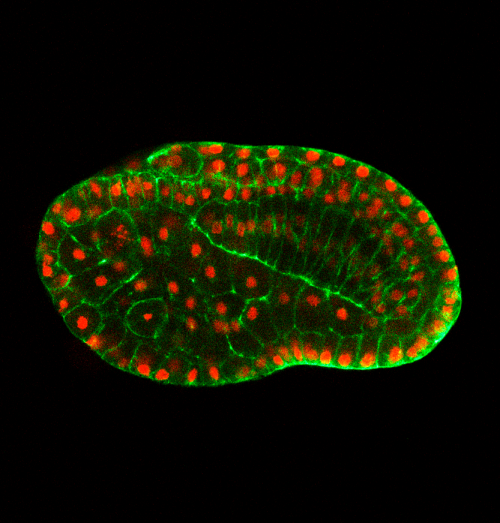
Can you give us the key results of the paper in a paragraph?
QL, YG, BD: In this paper, we first show that in the urochordate Ciona, embryonic tail bending is not dependent on the chorion, but rather is a self-organized and genetically programmed active process. We then found that actomyosin is asymmetrically accumulated at the ventral side of the notochord, and cell proliferation of the dorsal tail epidermis is faster than the ventral counterpart during bending. Through a combination of genetic perturbation and chemical drug manipulation, we reveal that both asymmetrical notochord contractility and differential epidermis proliferation are required for the tail-bending process. We further developed a model with experimentally measured parameters to simulate the bending process. The simulation result shows that the asymmetrical notochord contractility is sufficient to drive the tail bending, whereas the differential cell proliferation is a passive response to mechanical forces. Thus, we reveal a mechanism of asymmetrical notochord contractility coordinated with differential epidermis proliferation that drives embryonic bending. The main implications of this work are not only revealing that embryonic bending within the chorion is driven by intrinsic forces, but also demonstrating how the different tissues of the tail interact and coordinate to sculpt the embryonic shape.
Do you have any idea about what causes the ventral enrichment of actomyosin in the notochord?
QL, YG, BD: This is a really interesting question that is worthy of further investigation. We actually have screened some candidate signalling molecules using in situ hybridization, but have so far failed to get positive results. We knew from the published literature that some proteins, such as those in the extracellular matrix, also show polarity during notochord morphogenesis. Interestingly, during notochord convergent extension, the notochord preferentially accumulates laminin, a basement membrane marker, dorsally, and atypical protein kinase C, an apical cell polarity molecule, ventrally, which might provide a polarizing cue for polarized actomyosin enrichment.
Bending appears to be conserved with many other vertebrate and tunicate embryos: do you think it serves a particular purpose for the embryo? And is the mechanism you’ve discovered in Ciona likely to also be conserved?
QL, YG, BD: Von Baer’s laws say that vertebrate embryos converge on a common physical structure and hence show a similar morphology during early embryogenesis, called the phylotypic stages. For example, at the beginning of neurulation, chordate embryos are commonly C-shaped. We think that bending of the embryonic tail could help embryos elongate continuously within the chorion without mechanical damage. It definitely saves space to contain the elongated embryos within the chorion.
In this paper, we found that the polarized contractility of the notochord plays a major role in shaping the bending tail at early tailbud stages, whereas biased epidermal proliferation ensures the robustness of tail bending at later tailbud stages. However, our data did not rule out the possibility that other tissues and their interactions also contribute to embryonic tail bending. Indeed other data suggest that the role of the notochord in driving embryonic tail bending depends on the synergistic effect of other tissues. In more structurally-complex vertebrate systems, we really do not know whether notochord contractility still plays the active role: further investigations are definitely needed.
When doing the research, did you have any particular result or eureka moment that has stuck with you?
QL: I think the best moment for me was finding that actomyosin was temporarily enriched at the ventral side of the notochord. This interesting observation prompted us to investigate what role this polarised actomyosin might have in tail bending.
YG: A rational and effective model is only part of the way to success. The moments that most stick with me are when I find out a proper theory to depict the biological process. In this research, for example, we incorporated the active contraction of tissues into the model by using volumetric growth theory. Then it became easy to analyse the mechanical role of each tissue during tail bending.
And what about the flipside: any moments of frustration or despair?
QL: Sure, I definitely had a lot of them. For example, when we tried to confirm the role of the notochord in driving tail bending, our initial idea was to isolate the notochords from tailbud embryos and test whether they could bend spontaneously by the ventrally enriched actomyosin. This embryonic manipulation was rather challenging due to the tiny notochord located in the middle of the tail, surrounded by several tissues. We were stuck for a long time until we realized that we could try to think in an alternative way, and this transition led to the idea that physical modelling could also help us to address this question. Although I experienced so many failed attempts, I certainly learnt a lot from them, such as to always keep an open mind, and that interdisciplinary knowledge is essential for troubleshooting.
YG: As I am not a biology major, the technical terms sometimes become the obstacle to my understanding of the biological process. Likewise, my collaborators are not very good at mechanics and, at the first stage, it was a challenge to make the physical model understood. Fortunately, constant communication and discussions with my collaborators helped me overcome these difficulties.
Interdisciplinary knowledge is essential for troubleshooting.
What next for you after this paper?
QL: I am currently a postdoctoral fellow in the Umeå Centre for Molecular Medicine in Sweden, studying the neuronal basis of O2 sensing in C. elegans. I’m getting exposed to different fields, and I hope these interdisciplinary combinations will help me to explore more interesting questions.
YG: I will finish my dissertation in about six months. Meanwhile, I am looking for a postdoc position at present: the mechanical mechanisms underlying morphogenesis are intriguing and I hope to continue with this subject.
Where will this story take the Dong lab?
BD: Based on this and our previous work, we are recognizing the important roles of mechanical signalling in pattern formation during embryogenesis. For example, in this story, we believe that the faster cell proliferation in the dorsal midline epidermis can release the accumulated mechanical stress generated by asymmetrical notochord contractility. The follow up question is whether mechanochemical feedback exists between mechanical stretching and differential epidermis proliferation. If yes, how is the mechanical signalling sensed by the dorsal epidermis, and how does it respond?
Another question we are interested in pursuing is polarity signalling, which has important implications for tissue mechanics. Compared with the anatomically complex vertebrates, the Ciona embryonic tail is structurally simple, providing an excellent model to understand how polarity signalling impacts multi-tissue development.
Finally, let’s move outside the lab – what do you like to do in your spare time in Qingdao and Beijing?
QL: I spend most of my spare time either playing tennis, walking around our campus or climbing mountains nearby in Qingdao. These activities provide me a different kind of excitement outside of the lab, and more importantly, they enable me to balance my personal life and work.
YG: We are a big family in IBME. In our spare time, we often do sports together, like basketball and swimming. Besides, Beijing is an ancient and fascinating city, and I like to explore it with friends if we are free at weekends.
BD: I like to stay with my family during my spare time. We often climb Laoshan mountain and have weekend dinners together eating Qingdao’s delicious seafood. Sometimes, I also enjoy drinking Qingdao beer with my friends, which really can be relaxing, especially on summer nights.


 (No Ratings Yet)
(No Ratings Yet)



 (8 votes)
(8 votes)