Here are the highlights from the current issue of Development:
Dishevelled: the Notch-Wnt go-between
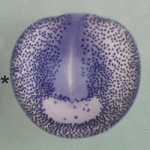 The Notch and Wnt signalling pathways are used during animal development to generate a diverse array of cell types. The two pathways often have opposing effects on cell-fate decisions but some cells receive inputs from both pathways simultaneously. In these circumstances, it is common for the receiving cell to exhibit a Wnt-ON/Notch-OFF response but how is this response generated? Now, on p. 4405, Keith Brennan and co-workers report that Wnt acts via Dishevelled, a key mediator of Wnt/β-catenin signalling, to inhibit the Notch pathway and that this crosstalk controls cell-fate specification during Xenopus epidermal development in vivo. Dishevelled, they report, binds and directly inhibits the CSL (CBF1, Suppressor of Hairless, Lag-1; RBPJκ in mice) transcription factors that mediate Notch signalling. Moreover, this crosstalk mechanism is conserved between vertebrates and invertebrates. Thus, by acting as both an activator of Wnt signalling and an inhibitor of Notch signalling, Dishevelled sharpens the distinction between opposing Wnt and Notch responses, thereby ensuring that robust cell-fate decisions are taken during development.
The Notch and Wnt signalling pathways are used during animal development to generate a diverse array of cell types. The two pathways often have opposing effects on cell-fate decisions but some cells receive inputs from both pathways simultaneously. In these circumstances, it is common for the receiving cell to exhibit a Wnt-ON/Notch-OFF response but how is this response generated? Now, on p. 4405, Keith Brennan and co-workers report that Wnt acts via Dishevelled, a key mediator of Wnt/β-catenin signalling, to inhibit the Notch pathway and that this crosstalk controls cell-fate specification during Xenopus epidermal development in vivo. Dishevelled, they report, binds and directly inhibits the CSL (CBF1, Suppressor of Hairless, Lag-1; RBPJκ in mice) transcription factors that mediate Notch signalling. Moreover, this crosstalk mechanism is conserved between vertebrates and invertebrates. Thus, by acting as both an activator of Wnt signalling and an inhibitor of Notch signalling, Dishevelled sharpens the distinction between opposing Wnt and Notch responses, thereby ensuring that robust cell-fate decisions are taken during development.
MicroRNA regulation of angiogenesis
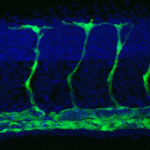 Secreted signalling molecules regulate cellular communication across tissues during development. For example, during angiogenesis in zebrafish embryos, vascular endothelial growth factor (VEGF) secreted by muscle controls the expansion and remodelling of the vascular network. The precise regulation of VEGF expression in muscle cells is therefore essential for angiogenesis but how is this regulation achieved? On p 4356, Yuichiro Mishima, Antonio Giraldez and colleagues report that miR-1 and miR-206 (miR-1/206; two conserved microRNAs with similar sequences) negatively regulate angiogenesis during zebrafish development. The negative effect of miR-1/206 on angiogenesis, the researchers report, is mediated in part by direct regulation of VegfAa in muscle. Thus, masking the target sites for miR-1/206 in the 3′ UTR of vegfaa has a pro-angiogenic effect similar to that of miR-1/206 knockdown. By contrast, reducing the levels of VegfAa rescues the increase in angiogenesis produced by miR-1/206 knockdown. Together, these findings uncover a novel function for miR-1/206 in the control of developmental angiogenesis and highlight a key role for microRNAs as regulators of cross-tissue signalling.
Secreted signalling molecules regulate cellular communication across tissues during development. For example, during angiogenesis in zebrafish embryos, vascular endothelial growth factor (VEGF) secreted by muscle controls the expansion and remodelling of the vascular network. The precise regulation of VEGF expression in muscle cells is therefore essential for angiogenesis but how is this regulation achieved? On p 4356, Yuichiro Mishima, Antonio Giraldez and colleagues report that miR-1 and miR-206 (miR-1/206; two conserved microRNAs with similar sequences) negatively regulate angiogenesis during zebrafish development. The negative effect of miR-1/206 on angiogenesis, the researchers report, is mediated in part by direct regulation of VegfAa in muscle. Thus, masking the target sites for miR-1/206 in the 3′ UTR of vegfaa has a pro-angiogenic effect similar to that of miR-1/206 knockdown. By contrast, reducing the levels of VegfAa rescues the increase in angiogenesis produced by miR-1/206 knockdown. Together, these findings uncover a novel function for miR-1/206 in the control of developmental angiogenesis and highlight a key role for microRNAs as regulators of cross-tissue signalling.
HIF1α controls collagen secretion under hypoxia
 The production of collagen – a major component of the extracellular matrix – depends on hydroxylation of proline residues, a reaction that uses molecular oxygen as substrate. Cells that develop in hypoxic settings can nevertheless produce collagen during embryogenesis. Elazar Zelzer and colleagues begin to resolve this paradox on p. 4473 by identifying the transcription factor hypoxia-inducible factor 1 α (HIF1α) as a central regulator of collagen hydroxylation and secretion by chondrocytes in the hypoxic growth plate of developing mouse bones. The researchers show that Hif1a loss of function in growth plate chondrocytes arrests the secretion of collagen. Hif1α, they report, drives the transcription of collagen prolyl 4-hydroxylase, which mediates collagen hydroxylation and its subsequent folding and secretion. Concurrently, Hif1α also maintains cellular oxygen levels, probably by controlling the expression of pyruvate dehydrogenase kinase 1, an inhibitor of the tricarboxylic acid cycle. This two-pronged mechanism, the researchers suggest, allows chondrocytes to secrete large amounts of collagen in the hypoxic environment of the growth plate.
The production of collagen – a major component of the extracellular matrix – depends on hydroxylation of proline residues, a reaction that uses molecular oxygen as substrate. Cells that develop in hypoxic settings can nevertheless produce collagen during embryogenesis. Elazar Zelzer and colleagues begin to resolve this paradox on p. 4473 by identifying the transcription factor hypoxia-inducible factor 1 α (HIF1α) as a central regulator of collagen hydroxylation and secretion by chondrocytes in the hypoxic growth plate of developing mouse bones. The researchers show that Hif1a loss of function in growth plate chondrocytes arrests the secretion of collagen. Hif1α, they report, drives the transcription of collagen prolyl 4-hydroxylase, which mediates collagen hydroxylation and its subsequent folding and secretion. Concurrently, Hif1α also maintains cellular oxygen levels, probably by controlling the expression of pyruvate dehydrogenase kinase 1, an inhibitor of the tricarboxylic acid cycle. This two-pronged mechanism, the researchers suggest, allows chondrocytes to secrete large amounts of collagen in the hypoxic environment of the growth plate.
Cycling towards sepal cell specification
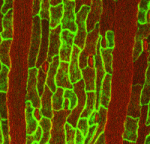 During development, cell division and cell specification must be coordinated to ensure that the tissues and organs of the adult organism are the correct size and contain the right proportions of various cell types. Here (p. 4416), Adrienne Roeder and colleagues investigate how developmental regulators interact with the cell cycle in Arabidopsis sepals to create a characteristic pattern of outer epidermal cells in which elongated giant cells, which are produced by endoreduplication (DNA replication without cell division), are interspersed between small cells, which divide mitotically. They show that distinct enhancers are expressed in giant cells and small cells, which suggests that these cells have different identities as well as different sizes and ploidies. Several members of the epidermal specification pathway control the identity of giant cells, they report, which is established upstream of cell-cycle regulation. By contrast, endoreduplication represses small cell identity. Thus, suggest the researchers, cell type affects cell-cycle regulation but, in addition, cell-cycle regulation can control cell identity.
During development, cell division and cell specification must be coordinated to ensure that the tissues and organs of the adult organism are the correct size and contain the right proportions of various cell types. Here (p. 4416), Adrienne Roeder and colleagues investigate how developmental regulators interact with the cell cycle in Arabidopsis sepals to create a characteristic pattern of outer epidermal cells in which elongated giant cells, which are produced by endoreduplication (DNA replication without cell division), are interspersed between small cells, which divide mitotically. They show that distinct enhancers are expressed in giant cells and small cells, which suggests that these cells have different identities as well as different sizes and ploidies. Several members of the epidermal specification pathway control the identity of giant cells, they report, which is established upstream of cell-cycle regulation. By contrast, endoreduplication represses small cell identity. Thus, suggest the researchers, cell type affects cell-cycle regulation but, in addition, cell-cycle regulation can control cell identity.
Epigenetic reprogramming egged on
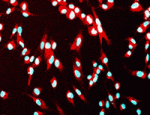 The generation of cloned embryos by somatic cell nuclear transfer (SCNT) into enucleated mature oocytes is inefficient because epigenetic reprogramming is limited in these embryos. However, treatment of somatic nuclei with a cytoplasmic extract from germinal vesicle (GV) stage oocytes before SCNT is known to improve the efficiency of cloned mouse production. Now (p. 4330), Hong-Thuy Bui, Jin-Hoi Kim and co-workers use an extract from GV stage pig oocytes (GVcyto-extract) to investigate epigenetic reprogramming events in pig fibroblasts. The researchers report that fibroblasts treated with GVcyto-extract express the stem cell-associated proteins Oct4 and Nanog and re-differentiate into three primary germ cell layers in vitro and in vivo. Moreover, the use of donor nuclei treated with GVcyto-extract, they report, increases the number of high quality SCNT-generated blastocysts that exhibit levels of histone H3-K9 methylation and acetylation and Oct4 and Nanog expression similar to those in embryos fertilised in vitro. These results suggest that a combination of epigenetic reprogramming techniques might improve the efficiency of development in SCNT-generated embryos.
The generation of cloned embryos by somatic cell nuclear transfer (SCNT) into enucleated mature oocytes is inefficient because epigenetic reprogramming is limited in these embryos. However, treatment of somatic nuclei with a cytoplasmic extract from germinal vesicle (GV) stage oocytes before SCNT is known to improve the efficiency of cloned mouse production. Now (p. 4330), Hong-Thuy Bui, Jin-Hoi Kim and co-workers use an extract from GV stage pig oocytes (GVcyto-extract) to investigate epigenetic reprogramming events in pig fibroblasts. The researchers report that fibroblasts treated with GVcyto-extract express the stem cell-associated proteins Oct4 and Nanog and re-differentiate into three primary germ cell layers in vitro and in vivo. Moreover, the use of donor nuclei treated with GVcyto-extract, they report, increases the number of high quality SCNT-generated blastocysts that exhibit levels of histone H3-K9 methylation and acetylation and Oct4 and Nanog expression similar to those in embryos fertilised in vitro. These results suggest that a combination of epigenetic reprogramming techniques might improve the efficiency of development in SCNT-generated embryos.
Notch pick and mix regulates lung development
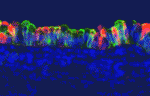 In developing mammalian lungs, the terminal buds of elongating airways contain multipotent epithelial progenitors that give rise to the three major lung epithelial cell types: Clara, ciliated and neuroendocrine (NE) cells. Now, Mitsuru Morimoto and colleagues provide new insights into how Notch signalling regulates the distribution of these epithelial cell types in the airway (see p. 4365). Using stepwise removal of Notch1, Notch2 and Notch3 from developing mouse lung epithelium, the researchers show that Notch2 alone mediates the Clara/ciliated cell fate decision whereas all three receptors regulate NE fate selection in an additive manner. All three Notch receptors also additively regulate the size of the presumptive pulmonary neuroepithelial body (pNEB; NEBs are clusters of NE cells) through mutual interactions between NE cells and a population of SSEA-1 (stage-specific antigen 1)-positive epithelial cells that surrounds the pNEB. These and other results indicate that two different assemblies of Notch receptors control the number and distribution of lung epithelial cell types during lung organogenesis.
In developing mammalian lungs, the terminal buds of elongating airways contain multipotent epithelial progenitors that give rise to the three major lung epithelial cell types: Clara, ciliated and neuroendocrine (NE) cells. Now, Mitsuru Morimoto and colleagues provide new insights into how Notch signalling regulates the distribution of these epithelial cell types in the airway (see p. 4365). Using stepwise removal of Notch1, Notch2 and Notch3 from developing mouse lung epithelium, the researchers show that Notch2 alone mediates the Clara/ciliated cell fate decision whereas all three receptors regulate NE fate selection in an additive manner. All three Notch receptors also additively regulate the size of the presumptive pulmonary neuroepithelial body (pNEB; NEBs are clusters of NE cells) through mutual interactions between NE cells and a population of SSEA-1 (stage-specific antigen 1)-positive epithelial cells that surrounds the pNEB. These and other results indicate that two different assemblies of Notch receptors control the number and distribution of lung epithelial cell types during lung organogenesis.
PLUS…
Drosophila neuroblasts: a model for stem cell biology
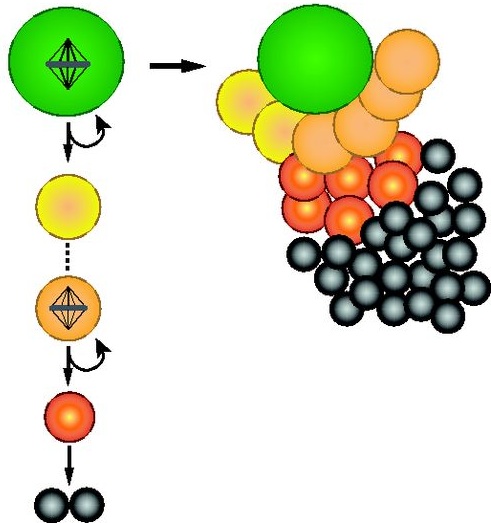 As part of the ‘Development: the big picture’ series, Homem and Knoblich explain why Drosophila neuroblasts, the stem cells of the developing fly brain, have emerged as a key model system for neural stem cell biology. See the Primer on p. 4297
As part of the ‘Development: the big picture’ series, Homem and Knoblich explain why Drosophila neuroblasts, the stem cells of the developing fly brain, have emerged as a key model system for neural stem cell biology. See the Primer on p. 4297
Endocytic receptor-mediated control of morphogen signaling
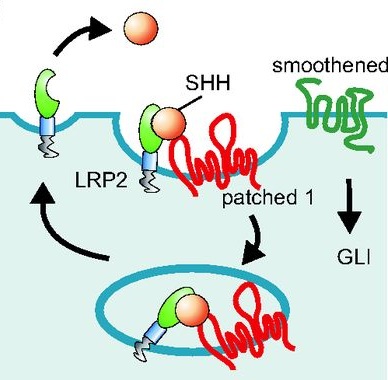 Thomas Willnow and colleaugues describe how low-density lipoprotein receptor-related proteins (LRPs) constitute central pathways that modulate morphogen presentation to target tissues and cellular signal reception, and how LRP dysfunction leads to developmental disturbances in many species. See the Primer on p. 4311
Thomas Willnow and colleaugues describe how low-density lipoprotein receptor-related proteins (LRPs) constitute central pathways that modulate morphogen presentation to target tissues and cellular signal reception, and how LRP dysfunction leads to developmental disturbances in many species. See the Primer on p. 4311
Piecing together the vertebrate skull
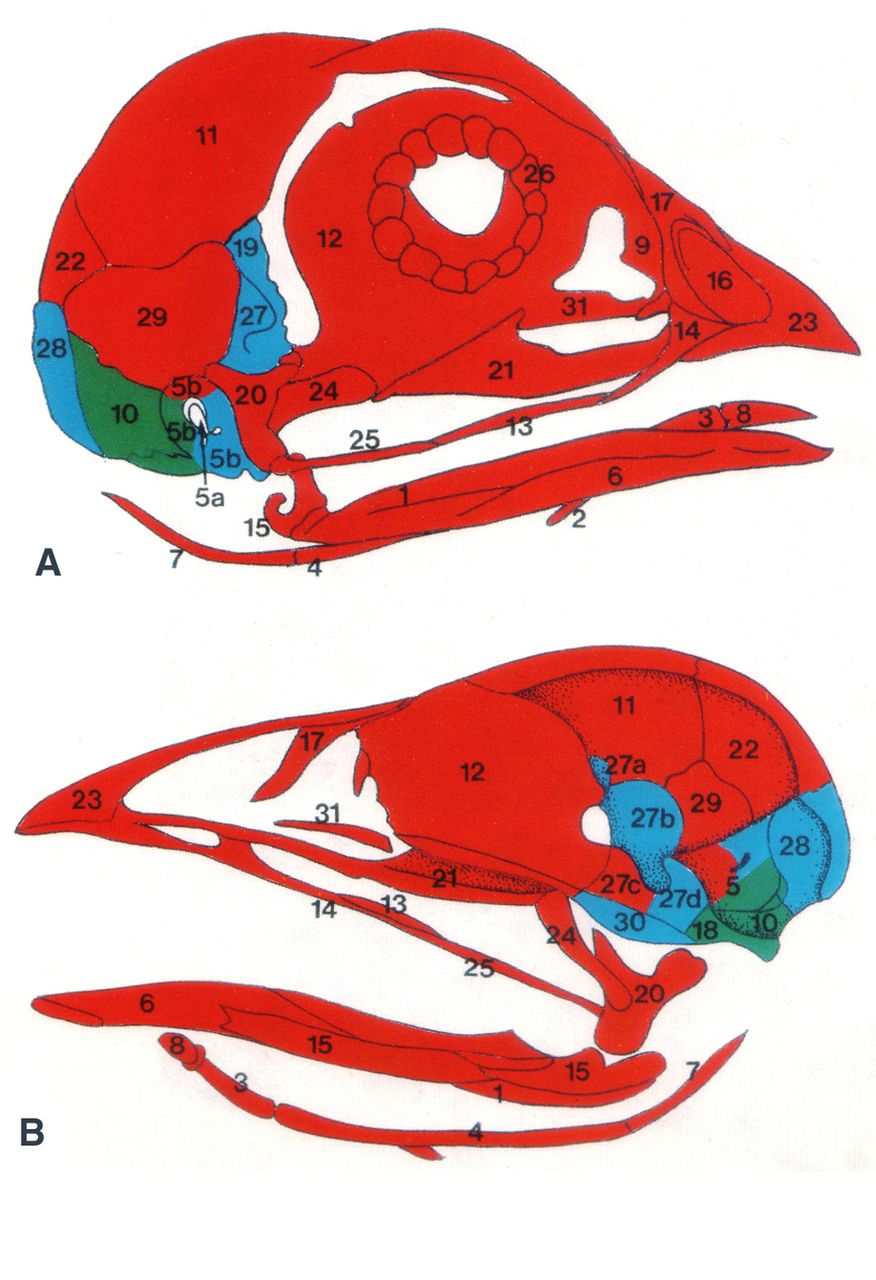 As part of the ‘Development Classics’ series, Nicole M. Le Douarin discusses her 1993 Development paper, in which she and her colleagues used the quail-chick chimera system to decipher the embryonic origin of the bones of the head skeleton of the avian embryo. See the Spotlight article on p. 4293
As part of the ‘Development Classics’ series, Nicole M. Le Douarin discusses her 1993 Development paper, in which she and her colleagues used the quail-chick chimera system to decipher the embryonic origin of the bones of the head skeleton of the avian embryo. See the Spotlight article on p. 4293
 (No Ratings Yet)
(No Ratings Yet)
 Loading...
Loading...


 (No Ratings Yet)
(No Ratings Yet) (11 votes)
(11 votes)
 (1 votes)
(1 votes) What was your favourite paper in the fields of developmental or stem cell biology this past year?
What was your favourite paper in the fields of developmental or stem cell biology this past year? The Notch and Wnt signalling pathways are used during animal development to generate a diverse array of cell types. The two pathways often have opposing effects on cell-fate decisions but some cells receive inputs from both pathways simultaneously. In these circumstances, it is common for the receiving cell to exhibit a Wnt-ON/Notch-OFF response but how is this response generated? Now, on p.
The Notch and Wnt signalling pathways are used during animal development to generate a diverse array of cell types. The two pathways often have opposing effects on cell-fate decisions but some cells receive inputs from both pathways simultaneously. In these circumstances, it is common for the receiving cell to exhibit a Wnt-ON/Notch-OFF response but how is this response generated? Now, on p.  Secreted signalling molecules regulate cellular communication across tissues during development. For example, during angiogenesis in zebrafish embryos, vascular endothelial growth factor (VEGF) secreted by muscle controls the expansion and remodelling of the vascular network. The precise regulation of VEGF expression in muscle cells is therefore essential for angiogenesis but how is this regulation achieved? On p
Secreted signalling molecules regulate cellular communication across tissues during development. For example, during angiogenesis in zebrafish embryos, vascular endothelial growth factor (VEGF) secreted by muscle controls the expansion and remodelling of the vascular network. The precise regulation of VEGF expression in muscle cells is therefore essential for angiogenesis but how is this regulation achieved? On p  The production of collagen – a major component of the extracellular matrix – depends on hydroxylation of proline residues, a reaction that uses molecular oxygen as substrate. Cells that develop in hypoxic settings can nevertheless produce collagen during embryogenesis. Elazar Zelzer and colleagues begin to resolve this paradox on p.
The production of collagen – a major component of the extracellular matrix – depends on hydroxylation of proline residues, a reaction that uses molecular oxygen as substrate. Cells that develop in hypoxic settings can nevertheless produce collagen during embryogenesis. Elazar Zelzer and colleagues begin to resolve this paradox on p.  During development, cell division and cell specification must be coordinated to ensure that the tissues and organs of the adult organism are the correct size and contain the right proportions of various cell types. Here (p.
During development, cell division and cell specification must be coordinated to ensure that the tissues and organs of the adult organism are the correct size and contain the right proportions of various cell types. Here (p.  The generation of cloned embryos by somatic cell nuclear transfer (SCNT) into enucleated mature oocytes is inefficient because epigenetic reprogramming is limited in these embryos. However, treatment of somatic nuclei with a cytoplasmic extract from germinal vesicle (GV) stage oocytes before SCNT is known to improve the efficiency of cloned mouse production. Now (p.
The generation of cloned embryos by somatic cell nuclear transfer (SCNT) into enucleated mature oocytes is inefficient because epigenetic reprogramming is limited in these embryos. However, treatment of somatic nuclei with a cytoplasmic extract from germinal vesicle (GV) stage oocytes before SCNT is known to improve the efficiency of cloned mouse production. Now (p.  In developing mammalian lungs, the terminal buds of elongating airways contain multipotent epithelial progenitors that give rise to the three major lung epithelial cell types: Clara, ciliated and neuroendocrine (NE) cells. Now, Mitsuru Morimoto and colleagues provide new insights into how Notch signalling regulates the distribution of these epithelial cell types in the airway (see p.
In developing mammalian lungs, the terminal buds of elongating airways contain multipotent epithelial progenitors that give rise to the three major lung epithelial cell types: Clara, ciliated and neuroendocrine (NE) cells. Now, Mitsuru Morimoto and colleagues provide new insights into how Notch signalling regulates the distribution of these epithelial cell types in the airway (see p.  As part of the ‘
As part of the ‘ Thomas Willnow and colleaugues describe how low-density lipoprotein receptor-related proteins (LRPs) constitute central pathways that modulate morphogen presentation to target tissues and cellular signal reception, and how LRP dysfunction leads to developmental disturbances in many species. See the Primer on p.
Thomas Willnow and colleaugues describe how low-density lipoprotein receptor-related proteins (LRPs) constitute central pathways that modulate morphogen presentation to target tissues and cellular signal reception, and how LRP dysfunction leads to developmental disturbances in many species. See the Primer on p.  As part of the ‘Development Classics’ series, Nicole M. Le Douarin discusses her 1993 Development paper, in which she and her colleagues used the quail-chick chimera system to decipher the embryonic origin of the bones of the head skeleton of the avian embryo. See the Spotlight article on p.
As part of the ‘Development Classics’ series, Nicole M. Le Douarin discusses her 1993 Development paper, in which she and her colleagues used the quail-chick chimera system to decipher the embryonic origin of the bones of the head skeleton of the avian embryo. See the Spotlight article on p.