October in preprints
Posted by the Node, on 6 November 2024
Welcome to our monthly trawl for developmental and stem cell biology (and related) preprints.
The preprints this month are hosted on bioRxiv and arXiv – use these links below to get to the section you want:
- Patterning & signalling
- Morphogenesis & mechanics
- Genes & genomes
- Stem cells, regeneration & disease modelling
- Plant development
- Environment, evolution and development
Research practice and education
Developmental biology
| Patterning & signalling
Jordann Lewis, Travis B. Lear, Brent Schlegel, Dominic Woods, Krithika Rao, Amy Sentis, Jay Tan, Rajaganapathi Jagannathan, Zaineb Javed, Aine N. Boudreau, Timothy Nelson, Mousumi Moulik, Nadine Hempel, Bill B. Chen, Sruti Shiva, Dhivyaa Rajasundaram, Toren Finkel, Anita Saraf
Pei-I Ku, Jamuna S Sreeja, Abhishek Chadha, David S Williams, Martin F Engelke, Radhika Subramanian
Maria Victoria Serrano, Stephanie Cottier, Lianzijun Wang, Sergio Moreira-Antepara, Anthony Nzessi, Zhiyu Liu, Byron Williams, Myeongwoo Lee, Roger Schneiter, Jun Liu
Aaron M. Savage, Alexandra C. Wagner, Ryan T. Kim, Paul Gilbert, Hani D. Singer, Erica Chen, Elane M. Kim, Noah Lopez, Kelly E. Dooling, Julia C. Paoli, S.Y. Celeste Wu, Sebastian Bohm, Rachna Chilambi, Tim Froitzheim, Steven J. Blair, Connor Powell, Adnan Abouelela, Anna G. Luong, Kara N. Thornton, Benjamin Tajer, Duygu Payzin-Dogru, Jessica L. Whited
Peggy P. Hsu, Ansley S. Conchola, Tristan Frum, Xiangning Dong, Lila Tudrick, Varun Ponnusamy, Michael S. Downey, Manqi Wu, Mengkun Yang, Yusoo Lee, Emma Niestroy, Yu-Hwai Tsai, Angeline Wu, Sha Huang, Ian A. Glass, Sofia D. Merajver, Jason R. Spence
EPHA2 Regulates SOX2 during Esophageal Development
Tianxia Li, Yosuke Mitani, Ricardo Cruz-Acuña, Tatiana A. Karaksheva, Varun Sahu, Cecilia Martin, Hiroshi Nakagawa, Joel Gabre
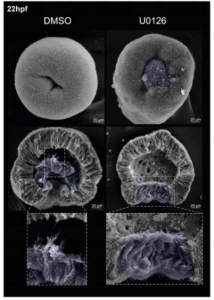
BMP signaling modulations control primitive streak patterning
Gaël Simon, Jean-Louis Plouhinec, Pascale Gilardi-Hebenstreit, Benoit Sorre, Jérôme Collignon
doi: https://doi.org/10.1101/2024.10.01.616050
Ling S. Loh, Joseph J. Hanly, Alexander Carter, Martik Chatterjee, Martina Tsimba, Donya N. Shodja, Luca Livraghi, Christopher R. Day, Robert D. Reed, W. Owen McMillan, Gregory A. Wray, Arnaud Martin
Connor Ross, Takuya Azami, Marika Salonna, Richard Gyuris, Jennifer Nichols, Stefan Hoppler
Cellular signalling protrusions enable dynamic distant contacts in spinal cord neurogenesis
Joshua Hawley, Robert Lea, Veronica Biga, Nancy Papalopulu, Cerys Manning
MMP21 behaves as a fluid flow transported morphogen to impart laterality during development
Tim Ott, Amelie Brugger, Emmanuelle Szenker-Ravi, Yvonne Kurrle, Olivia Aberle, Matthias Tisler, Martin Blum, Sandra Whalen, Patrice Bouvagnet, Bruno Reversade, Axel Schweickert
Independent control of neurogenesis and dorsoventral patterning by NKX2-2
Sumin Jang, Elena Abarinov, Julie Dobkin, Hynek Wichterle
Abigail E. Descoteaux, Marko Radulovic, Dona Alburi, Cynthia A. Bradham
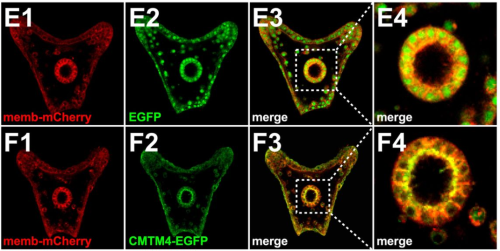
Evidence for strong cell-scale signalling during planar polarisation in the Drosophila wing
Alexandre Carayon, David Strutt
Shh signaling directs dorsal ventral patterning in the regenerating X. tropicalis spinal cord
Avery Angell Swearer, Samuel Perkowski, Andrea Wills
Cluster Assembly Dynamics Drive Fidelity of Planar Cell Polarity Polarization
Silas Boye Nissen, Alexis T. Weiner, Kaye Suyama, Pablo Sanchez Bosch, Song Song, Yuan Gu, Alexander R. Dunn, Jeffrey D. Axelrod
Capicua maintains anterior-posterior axis in Blattella germanica ovaries
Nashwa Elshaer, Jorge Escudero, Maria-Dolors Piulachs
Shc1 cooperates with Frs2 and Shp2 to recruit Grb2 in FGF-induced lens development
Qian Wang, Hongge Li, Yingyu Mao, Ankur Garg, Eun Sil Park, Yihua Wu, Alyssa Chow, John Peregrin, Xin Zhang
Characterization of Hippo Signaling Components in the Early Dorsal Pancreatic Bud
Neha Ahuja, Caitlin Maynard, Tyler Bierschenck, Ondine Cleaver
Yuchen Liu, Tianli Qin, Xin Weng, Bernice Leung, Karl Kam Hei So, Boshi Wang, Wanying Feng, Alexander Marsolais, Sheena Josselyn, Pingbo Huang, Bernd Fritzsch, Chi-Chung Hui, Mai Har Sham
Zhiling Zhao, Rieko Asai, Takashi Mikawa
Emmanuel Haillot, Tatiana Lebedeva, Julia Steger, Grigory Genikhovich, Juan D. Montenegro, Alison G. Cole, Ulrich Technau
| Morphogenesis & mechanics
Morphomechanic tuning of ERK by actin-TFII-IΔ regulates cell identity
Qiao Wu, Jian Zhang, Bing Long, Xiao Hu, Bruna Mafra de Faria, Stephen Maxwell Scalf, Kutay Karatepe, Wenxiang Cao, Nikolaos Tsopoulidis, Andres Binkercosen, Masaki Yagi, Aaron Weiner, Mary Kaileh, Enrique M. De La Cruz, Ananda L Roy, Konrad Hochedlinger, Shangqin Guo
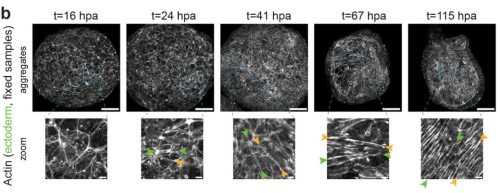
Anisotropic stretch biases the self-organization of actin fibers in multicellular Hydra aggregates
Anaïs Bailles, Giulia Serafini, Heino Andreas, Christoph Zechner, Carl Modes, Pavel Tomancak
Carole Luthold, Marie Didion, Emilio Benedum, Ann-Kathrin Burkhart, Nina Demmerle, Gubesh Gunaratnam, Vanessa Samira Rácz, Markus Bischoff, Annika Ridzal, Sandra Iden
Thea Jacobs, Jone Isasti Sanchez, Steven Reger, Stefan Luschnig
Anna Jaeschke, Matt S. Hepburn, Alireza Mowla, Brendan F. Kennedy, Chii Jou Chan
Si Chen, Isabella Burda, Purvil Jani, Bex Pendrak, Meredith N. Silberstein, Adrienne H.K. Roeder
Mechanical control of germ cell specification in Arabidopsis anthers
Chan Liu, Hui Shi, Yuting Han, Pan Wang, Kexin Li, Zhishuai Zhang, Jiazheng Liu, Yafeng Zheng, Linlin Li, Limei Lin, Chen Liang, Binjun Qin, Hua Han, Shunong Bai, Xiao Liu, Wenqian Chen, Feng Zhao
Spatiotemporal dynamics of primary and motile cilia throughout lung development
Stephen Spurgin, Ange Michelle Nguimtsop, Fatima N. Chaudhry, Sylvia N. Michki, Jocelynda Salvador, M. Luisa Iruela-Arispe, Jarod A. Zepp, Saikat Mukhopadhyay, Ondine Cleaver
IGF2 mediates Hippo signaling to control liver size
Zhenxing Zhong, Ruxin Jin, Yiting Zhong, Li Zhang, Deqian Chen, Zhihan Jiao, Fanhui Zhou, Rui Zhu, Jian Wu, Rui Dong, Kuiran Dong, Fei Lan, Yu Wang, Kun-Liang Guan, Fa-Xing Yu
Mechanical regulation of tissue flatness in Marchantia
Jordan Ferria, Carla J.A. Fournié, Magdalena H. Jankowska, Doron Grossman, Adrienne H.K. Roeder, Stéphanie Drevensek, Arezki Boudaoud
A mitochondrial redox switch licenses the onset of morphogenesis in animals
Updip Kahlon, Francesco Dalla Ricca, Saraswathi J. Pillai, Marine Olivetta, Kevin M. Tharp, Li-En Jao, Omaya Dudin, Kent McDonald, Mustafa G. Aydogan
Mechanochemical Patterning Localizes the Organizer of a Luminal Epithelium
Sera Lotte Weevers, Alistair D. Falconer, Moritz Mercker, Hajar Sadeghi, Jaroslav Ferenc, Albrecht Ott, Dietmar B. Oelz, Anna Marciniak-Czochra, Charisios D. Tsiairis
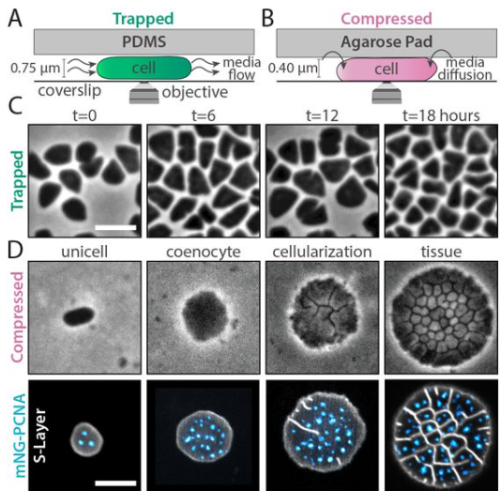
Tissue-Like Multicellular Development Triggered by Mechanical Compression in Archaea
Theopi Rados, Olivia S. Leland, Pedro Escudeiro, John Mallon, Katherine Andre, Ido Caspy, Andriko von Kügelgen, Elad Stolovicki, Sinead Nguyen, Inés Lucía Patop, Thiberio Rangel, Sebastian Kadener, Lars D. Renner, Vera Thiel, Yoav Soen, Tanmay A.M. Bharat, Vikram Alva, Alex Bisson
| Genes & genomes
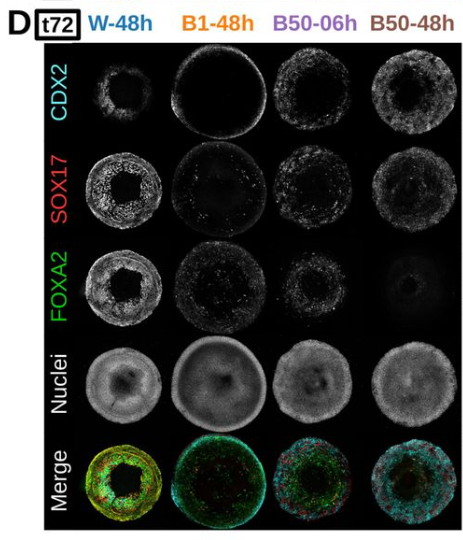
Khusali Gupta, Ping Xu, Judith Gallant, Yeonsoo Yoon, Jaime A. Rivera-Pérez, Jeanne B. Lawrence
doi: https://doi.org/10.1101/2024.09.29.615605
Nora Ditzer, Ezgi Senoglu, Theresa M. Schütze, Aikaterina Nikolaidi, Annika Kolodziejczyk, Katrin Sameith, Sevina Dietz, Razvan P. Derihaci, Cahit Birdir, Anne Eugster, Mike O. Karl, Andreas Dahl, Pauline Wimberger, Franziska Baenke, Claudia Peitzsch, Mareike Albert
Yangqi Su, Evguenia Kouranova, Jonathan Shea, Xiaoxia Cui, Zhengchang Su
M. Fernanda Palominos, Vanessa Muhl, Christopher H. Martin
Audrey J. Marsh, Sergei Pirogov, Abby J. Ruffridge, Suresh Sajwan, Tyler J. Gibson, George Hunt, Yadwinder Kaur, Melissa M. Harrison, Mattias Mannervik
William F Beckman, Lydia M Parkinson, Lewis Chaytor, Anna Philpott
A piRNA regulating oogenesis and embryo development in cockroaches
Judit Gonzalvo, Nuria Farrus, Jorge Escudero, David Pujal, Josep Bau, Maria-Dolors Piulachs
Deciphering gene regulatory programs in mouse embryonic skin through single-cell multiomics analysis
Qiuting Deng, Pengfei Cai, Yingjie Luo, Zhongjin Zhang, Wen Ma, Zijie Huang, Xiaoya Chen, Shijie Hao, Weiguang Ma, Jiangshan Xu, Mengnan Cheng, Xiumei Lin, Ru Zhou, Shanshan Duan, Junjie Chen, Ronghai Li, Xuyang Shi, Chang Liu, Peng Gao, Jianting Li, Jun Xie, Longqi Liu, Yue Yuan, Chuanyu Liu
Madison James Yang, Kamilla Sedov, Max Y. Chen, Faria Zafar, Birgitt Schüle
Dimitris Botskaris, Ioannis K. Deligiannis, Ioanna Peraki, Haroula Kontaki, Marianna Stagaki, Matthieu D. Lavigne, Celia P. Martinez-Jimenez, Iannis Talianidis
Surbhi Sood, Aktan Alpsoy, Guanming Jiao, Alisha Dhiman, Charles Samuel King, Gabriella Grace Conjelko, Judy E. Hallett, Sagar M Utturkar, Jill E Hutchcroft, Emily C Dykhuizen
Amber M. Ridgway, Javier Figueras Jimenez, Maria D. S. Nunes, Alistair P. McGregor
Contextualising transcription factor binding during embryogenesis using natural sequence variation
Olga M. Sigalova, Mattia Forneris, Frosina Stojanovska, Bingqing Zhao, Rebecca R. Viales, Adam Rabinowitz, Fayrouz Hamal, Benoît Ballester, Judith B Zaugg, Eileen E.M. Furlong
Komal Yasmin, Tatyana B Nesterova, Neil Brockdorff
Saeko Takada, Bonnie J. Bolkan, MaryJane O’Connor, Michael Goldberg, Michael B. O’Connor
Hiroki Tsutsumi, Tomoki Chiba, Yuta Fujii, Takahide Matsushima, Tsuyoshi Kimura, Akinori Kanai, Akio Kishida, Yutaka Suzuki, Hiroshi Asahara
Foxn3 is part of a transcriptional network that regulates cilia genes in the developing mouse retina
Huanqing Zhang, Fan Meng, David L. Turner
Hao Ming, Rajan Iyyappan, Kianoush Kakavand, Michal Dvoran, Andrej Susor, Zongliang Jiang
| Stem cells, regeneration & disease modelling
Axolotl epigenetic clocks offer insights into the nature of negligible senescence
Yuliia Haluza, Joseph A. Zoller, Ake T. Lu, Hannah E. Walters, Martina Lachnit, Robert Lowe, Amin Haghani, Robert T. Brooke, Naomi Park, Maximina H. Yun, Steve Horvath
Lgl resets Par complex membrane loading at mitotic exit to polarize neural stem cells
Bryce LaFoya, Sarah E. Welch, Kenneth E. Prehoda
RO8191, a new compound for initiating embryo implantation in mice
Junlan Shu, Jumpei Terakawa, Satoko Osuka, Ayako Muraoka, Jiali Ruan, Junya Ito, Atsuo Iida, Eiichi Hondo
Liver Sinusoidal Endothelial Cells and Laminin dictate cholangiocytes’ fate in chronic liver disease
Rita Manco, Camilla Moliterni, Gauthier Neirynck, Maxime De Rudder, Corinne Picalausa, Leana Ducor, Montserrat Fraga, Frédéric Lemaigre, Christine Sempoux, Alexandra Dili, Isabelle A. Leclercq
Yue Lu, Tezin Walji, Pratima Pandey, Chuanli Zhou, Christa Whelan Habela, Scott Snapper, Rong Li, Elizabeth Chen
Shiri Kult Perry, Nikko-Ideen Shaidani, Marko E Horb, Neil Shubin
Injury-induced transcription in the planarian outer epithelium is critical for tissue regeneration
Pallob Barai, Mariya S. Kibtiya, Nathan G. Maggard, Shishir Biswas, Elizabeth M. Duncan
Carolyn Sangokoya, Robert Blelloch
Azali Azlan, Li Zhu, Ryuya Fukunaga
A post-mitotic in vitro murine as a model of muscle damage and repair
Angelo Galluccio, Samantha Maurotti, Francesca Rita Noto, Francesca Scionti, Carmelo Pujia, Elisa Mazza, Yvelise Ferro, Rosario Mare, Nadia Geirola, Bernadette Scopacasa, Patrizio Candeloro, Luca Tirinato, Angela Sciacqua, Arturo Pujia, Stefano Romeo, Tiziana Montalcini
Yingnan Lei, Mai Chi Duong, Nuša Krivec, Charlotte Janssens, Marius Regin, Anfien Huyghebaert, Edouard Couvreu de Deckersberg, Karen Sermon, Diana Al Delbany, Claudia Spits
Julian Weihs, Fatima Baldo, Alessandra Cardinali, Gehad Youssef, Katarzyna Ludwik, Harald Stachelscheid, Nils Haep, Peter Tang, Igor Sauer, Pavitra Kumar, Cornelius Engelmann, Susanna Quach, Philip Bufler, Namshik Han, Milad Rezvani
Ryosuke Isotani, Masaki Igarashi, Masaomi Miura, Kyoko Naruse, Satoshi Kuranami, Manami Katoh, Seitaro Nomura, Toshimasa Yamauchi
Milad Rezvani, Kyle Lewis, Susanna Quach, Kentaro Iwasawa, Julian Weihs, Hasan Al Reza, Yuqi Cai, Masaki Kimura, RanRan Zhang, Yuka Milton, Praneet Chaturvedi, Konrad Thorner, Ramesh C. Nayak, Jorge Munera, Phillip Kramer, Brian R. Davis, Appakalai N. Balamurugan, Yeni Ait Ahmed, Marcel Finke, Rose Yinghan Behncke, Adrien Guillot, René Hägerling, Julia K. Polansky, Philip Bufler, Jose A Cancelas, James M. Wells, Momoko Yoshimoto, Takanori Takebe
STAT3 signalling enhances tissue expansion during postimplantation mouse development
Takuya Azami, Bart Theeuwes, Mai-Linh N Ton, William Mansfield, Luke Harland, Masaki Kinoshita, Berthold Gottgens, Jennifer Nichols
Léa Torcq, Catherine Vivier, Sandrine Schmutz, Yann Loe-Mie, Anne A. Schmidt
A limbal stem cell deficiency murine model with residual limbal stem cells
Hideaki Someya, Shintaro Shirahama, Margarete M. Karg, Meredith S. Gregory-Ksander, Reza Dana, Bruce R. Ksander
B. Pardo-Rodríguez, A.M. Baraibar, I. Manero-Roig, J. Luzuriaga, J. Salvador-Moya, Y. Polo, R. Basanta-Torres, F. Unda, S. Mato, G. Ibarretxe, J.R. Pineda
Loic Fort, Wenjun Wang, Ian Macara
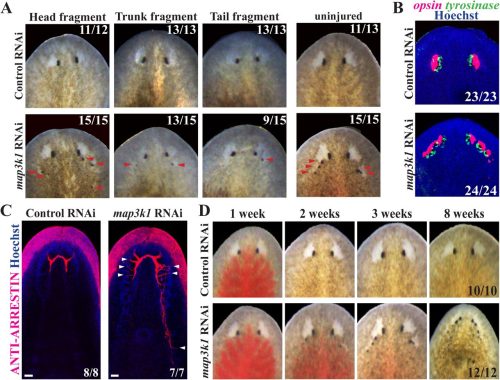
map3k1 suppresses terminal differentiation of migratory eye progenitors in planarian regeneration
Katherine C. Lo, Christian P. Petersen
Local control of cellular proliferation underlies neuromast regeneration in zebrafish
Natalia G. Lavalle, Jerónimo Miranda-Rodríguez, Emanuel Cura Costa, Augusto Borges, Oriol Viader-Llargués, Hernán López-Schier, Osvaldo Chara
Carson Shalaby, James Garifallou, Christopher S Thom
The PUF RNA-binding protein, FBF-2, maintains stem cells without binding to RNA
Brian H. Carrick, Sarah L. Crittenden, MaryGrace Linsley, Stephany J. Costa Dos Santos, Marvin Wickens, Judith Kimble
Jessica Ensing, Amber D. Ide, Carla Gilliland, Visakuo Tsurho, Isabella Caza, Amber N. Stratman, Nathan J. Lanning, Stephanie Grainger
| Plant development
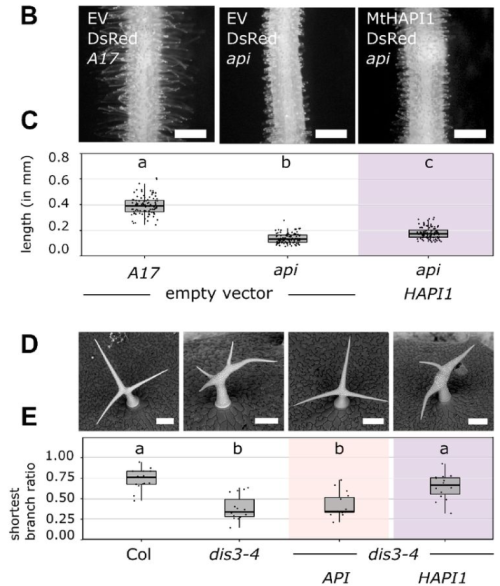
A molecular basis for plant SCAR/WAVE functional divergence
Sabine Brumm, Aleksandr Gavrin, Matthew Macleod, Guillaume Chesneau, Annika Usländer, Sebastian Schornack
Zahit Kaya, Amir Maqbool, Motofumi Suzuki, Emre Aksoy
Stochastic Gene Expression in Auxin Signaling in the Floral Meristem of Arabidopsis thaliana
Shuyao Kong, Mingyuan Zhu, Adrienne H.K. Roeder
NPF4.1 imports embryo-derived GA4 to the endosperm to promote seed germination
Mathilde Sirlin-Josserand, Lali Sakvarelidze-Achard, David Pflieger, Jean-Michel Davière, Patrick Achard
Sara Cannavò, Chiara Paleni, Alma Costarelli, Maria Cristina Valeri, Martina Cerri, Antonietta Saccomanno, Veronica Gregis, Graziella Chini Zittelli, Petre I. Dobrev, Lara Reale, Martin M. Kater, Francesco Paolocci
The genetic basis of replicated bullseye pattern reduction across the Trionum Complex
May T. S. Yeo, Alice L. M. Fairnie, Valentina Travaglia, Joseph F. Walker, Lucie Riglet, Selin Zeyrek, Edwige Moyroud
PRC2 facilitates the transition from heterotrophy to photoautotrophy during seedling emergence
Naseem Samo, María Guadalupe Trejo-Arellano, Lenka Gahurová, Alexander Erban, Alina Ebert, Quentin Rivière, Jiří Kubásek, Fatemeh Aflaki, Helena Hönig Mondeková, Armin Schlereth, Annick Dubois, Mingxi Zhou, Ondřej Novák, Jiří Šantrůček, Daniel Bouyer, Franҫois Roudier, Joachim Kopka, Iva Mozgová
Katelin M. Burow, Xi Yang, Yun Zhou, Brian P. Dilkes, Jennifer H. Wisecaver
ERECTA family signaling controls cell fate specification during ovule initiation in Arabidopsis
Alex M. Overholt, Christina Elaine Pierce, Calen Seth Paleologos, Elena D. Shpak
Shaping Kale Morphology and Physiology Using Different LED Light Recipes
Sabine Scandola, Lauren E. Grubb, Brigo Castillo, Lexyn Iliscupidez, Curtis Kennedy, Nicholas Boyce, R. Glen Uhrig
Highly expressed cell wall genes contribute to robustness of sepal size
Diego A. Hartasánchez, Mathilde Dumond, Nelly Dubrulle, Françoise Monéger, Arezki Boudaoud
Yang Liu, Valentin Joly, Mohamed Sabar, Daniel Philippe Matton, David Morse
| Environment, evolution and development
Alicia Tovar, Scott Monahan, Trevor Mugoya, Adrian Kristan, Walker Welch, Ryan Dettmers, Camila Arce, Theresa Buck, Michele Ruben, Alexander Rothenberg, Roxane Saisho, Ryan Cartmill, Timothy Skaggs, Robert Reyes, MJ Lee, John Obrycki, William Kristan, Arun Sethuraman
Methylomes reveal recent evolutionary changes in populations of two plant species
Kevin Korfmann, Andreas Zauchner, Aurélien Tellier, Ramesh Arunkumar
Polar bodies serve as a landmark for anteroposterior axis formation in spiders
Ruixun Wang, Matthias Pechmann
Evolutionary Insights into Muscle Fiber Distribution in the Twin Tails of Ornamental Goldfish
Kinya G Ota, Gembu Abe, Chen-Yi Wang, Ing-Jia Li, Paul Gerald Layague Sanchez, Tzu-Chin Chi
Christian J. Bellissimo, Tatiane A. Ribeiro, Erica Yeo, Patrycja A. Jazwiec, Howard Luo, Jaskiran Bains, Deborah M. Sloboda
Cenozoic evolutionary history obscures the Mesozoic origins of acanthopterygian fishes
Chase D. Brownstein, Alex Dornburg, Thomas J. Near
EJ Huang, Jeeun Parksong, Amy F. Peterson, Fernando Torres, Sergi Regot, Gabriel S. Bever
Diversity and evolution of Radiolaria: Beyond the stars of the ocean
Miguel M. Sandin, Johan Renaudie, Noritoshi Suzuki, Fabrice Not
Growth compensation upon changes in tissue size in the Drosophila abdomen
Ana Ferreira, Andrea Cairoli, Federica Mangione, Maxine V. Holder, Anna Ainslie, Birgit L. Aerne, Guillaume Salbreux, Nicolas Tapon
Evolution of the non-visual and visual opsin gene repertoire in ray-finned fishes
Maxime Policarpo, Lily G. Fogg, Fabio Cortesi, Walter Salzburger
Vinicius Delgado da Rocha, Everton Geraldo Capote Ferreira, Fernanda Machado Castanho, Marcia Kamogae Kuwahara, Cláudia Vieira Godoy, Maurício Conrado Meyer, Kerry F. Pedley, Ralf T. Voegele, Anna Lipzen, Kerrie Barry, Igor V. Grigoriev, Marco Loehrer, Ulrich Schaffrath, Catherine Sirven, Sebastien Duplessis, Francismar Corrêa Marcelino-Guimarães
Moisès Bernabeu, Saioa Manzano-Morales, Toni Gabaldón
Evolution of plant cell-type-specific cis-regulatory elements
Haidong Yan, John P. Mendieta, Xuan Zhang, Alexandre P. Marand, Yan Liang, Ziliang Luo, Mark A.A. Minow, Hosung Jang, Xiang Li, Thomas Roulé, Doris Wagner, Xiaoyu Tu, Yonghong Wang, Daiquan Jiang, Silin Zhong, Linkai Huang, Susan R. Wessler, Robert J. Schmitz
Germplasm stability in zebrafish requires maternal Tdrd6a and Tdrd6c
Alessandro Consorte, Yasmin El Sherif, Fridolin Kielisch, Nadine Wittkopp, René F. Ketting
Genetic variation in male mate choice for large females in Drosophila melanogaster
Grace S. Freed, Isabella G. Martinez, Avigayil Lev, Ana-Maria Anthony Cuadrado, Alison Pischedda
Deep-sea fish reveal alternative pathway for vertebrate visual development
Lily G. Fogg, Stamatina Isari, Jonathan E. Barnes, Jagdish Suresh Patel, N. Justin Marshall, Walter Salzburger, Fabio Cortesi, Fanny de Busserolles
Allyson Caldwell, Liheng Yang, Elizabeth A. Scheef, Amitinder Kaur, Carolyn B. Coyne
Laura Wögler, Christoph Kurze
London C. Mitchell, Armin P. Moczek, Erica M. Nadolski
Identification of a specialized lipid barrier for Drosophila metamorphosis
Lena Lampe, Clare L. Newell, Bing-Jun Wang, Rami Makki, Cyrille Alexandre, Ian S. Gilmore, Li Zhao, Alex P. Gould
Magdalena Schindler, Christian Feregrino, Silvia Aldrovandi, Bai-Wei Lo, Anna A. Monaco, Alessa R. Ringel, Ariadna Morales, Tobias Zehnder, Rose Yinghan Behncke, Juliane Glaser, Alexander Barclay, Guillaume Andrey, Bjørt K. Kragesteen, René Hägerling, Stefan Haas, Martin Vingron, Igor Ulitsky, Marc Marti-Renom, Julio Hechavarria, Nicolas Fasel, Michael Hiller, Darío Lupiáñez, Stefan Mundlos, Francisca M. Real
Kim-Sara Wagner, Frédéric Salasc, Silke-Mareike Marten, Olivia Roth
Hope M. Healey, Hayden B. Penn, Clayton M. Small, Susan Bassham, Vithika Goyal, Micah A. Woods, William A. Cresko
Oxygen level alters energy metabolism in bovine preimplantation embryos
N. Boskovic, M. Ivask, G. Yazgeldi Gunaydin, B. Yaşar, S. Katayama, A. Salumets, T. Org, A. Kurg, K. Lundin, T. Tuuri, C. O. Daub, J. Kere
Hybrid incompatibility emerges at the one-cell stage in interspecies Caenorhabditis embryos
Jessica Bloom, Rebecca Green, Arshad Desai, Karen Oegema, Scott A. Rifkin
Evolution of maternal and early zygotic transcript regulation across Drosophila
Charles S. Omura, Susan E. Lott
Ceri J. Weber, Alexander J. Weitzel, Alexander Y. Liu, Erica G. Gacasan, Robert L. Sah, Kimberly L. Cooper
Planarians Develop Radiotolerance to Recurrent Ionizing Radiation Exposure
Paul G. Barghouth, Benjamin Ziman, Eli Isael Maciel, Peter Karabinis, Salvador Rojas, Natasha M. Flores, Edelweiss Pfister, Néstor J. Oviedo
Shifts in embryonic oxygen levels cue heterochrony in limb initiation
Meng Zhu, Rinaldo Catta-Preta, ChangHee Lee, Clifford Tabin
Kumi Matsuura-Tokita, Ayaka Sakai, Takamasa Suzuki, Akihiko Nakano, Tetsuya Higashiyama
Cell Biology
Cristofer Calvo, Casey O. Swoboda, Fabian Montecino Morales, Siddhant Nagar, Michael J. Petrany, Chengyi Sun, Hima Bindu Durumutla, Mattia Quattrocelli, Douglas P. Millay
The role of cytochrome c in mitochondrial metabolism of human oocytes
Jakub Maciej Surmacki, Halina Abramczyk, Bogna Sobkiewicz, Renata Walczak-Jędrzejowska, Jolanta Słowikowska-Hilczer, Katarzyna Marchlewska
The molecular chronology of mammary epithelial cell fate switching
Queralt Vallmajo-Martin, Zhibo Ma, Sumana Srinivasan, Divya Murali, Christopher Dravis, Kavitha Mukund, Shankar Subramaniam, Geoffrey M. Wahl, Nikki K. Lytle
Enhanced RNA-targeting CRISPR-Cas technology in zebrafish
Ismael Moreno-Sanchez, Luis Hernandez-Huertas, Daniel Nahon-Cano, Carlos Gomez-Marin, Pedro Manuel Martinez-García, Anthony J. Treichel, Laura Tomas-Gallardo, Gabriel da Silva Pescador, Gopal Kushawah, Alejandro Díaz-Moscoso, Alejandra Cano-Ruiz, John A. Walker II, Manuel J. Muñoz, Kevin Holden, Joan Galcerán, María Ángela Nieto, Ariel Bazzini, Miguel A. Moreno-Mateos
Intra-manchette transport employs both microtubule and actin tracks
Jo H. Judernatz, Laura Pérez Pañeda, Tereza Kadavá, Albert J. R. Heck, Tzviya Zeev-Ben-Mordehai
Visualizing developmental dynamics of nuclear morphology and transport machinery in Drosophila
Yuki Shindo, Shruthi Balachandra, Amanda A. Amodeo
Mammary Epithelial Migration is EMT-Independent
Jing Chen, Rongze ma, Zhixuan Deng, Yunzhe Lu, Jiecan Zhou, Kun Xia, Ophir D. Klein, Pengfei Lu
An interkinetic envelope surrounds chromosomes between meiosis I and II in C. elegans oocytes
Layla El Mossadeq, Laura Bellutti, Rémi Le Borgne, Julie C. Canman, Lionel Pintard, Jean-Marc Verbavatz, Peter Askjaer, Julien Dumont
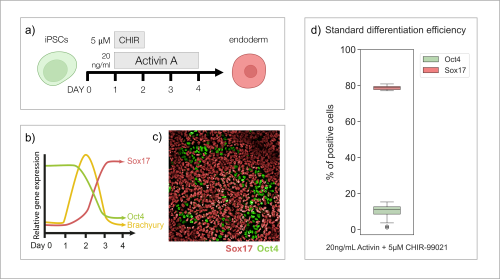
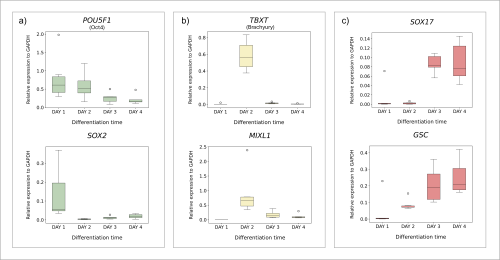
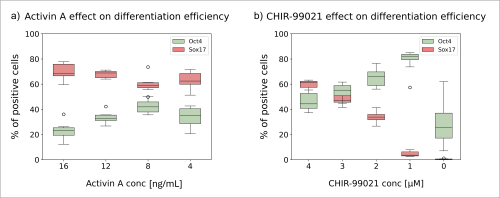
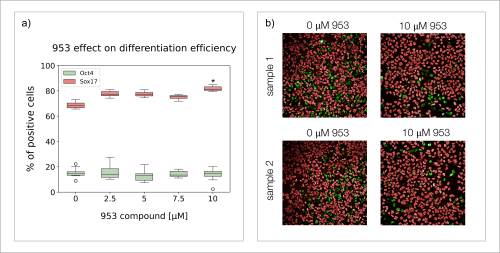
Nathaniel J. Hogrebe, Mason D. Schmidt, Punn Augsornworawat, Sarah E. Gale, Mira Shunkarova, Jeffrey R. Millman
Kiran Suhas Nilangekar, Bhupendra V. Shravage
Mitochondrial fission controls astrocyte morphogenesis and organization in the cortex
Maria Pia Rodriguez Salazar, Sprihaa Kolanukuduru, Valentina Ramirez, Boyu Lyu, Gabrielle Sejourne, Hiromi Sesaki, Guoqiang Yu, Cagla Eroglu
Sara Di Carlo, Adrian Salas-Bastos, Mariela Castelblanco Castelblanco, Muriel Auberson, Marie Rumpler, Malaury Tournier, Lukas Sommer, Olaia Naveiras, Edith Hummler
Bryce LaFoya, Kenneth E. Prehoda
eIF4ET regulates meiotic proteome levels to enable oocyte formation and storage
Priyankaa Bhatia, Ruchi Amin, Nicole E. Familiari, Kan Yaguchi, Vanna M. Tran, Alec Bond, Orhan Bukulmez, Jeffrey B. Woodruff
Human spermatogenesis leads to a reduced nuclear pore structure and function
Ália dos Santos, Oliver Knowles, Tom Dendooven, Thomas Hale, Alister Burt, Piotr Kolata, Giuseppe Cannone, Dom Bellini, David Barford, Matteo Allegretti
Modelling
Daisy Ulloa, Kelsey M. Temple, Theresa M. Casey, Uduak Z George
Quantitative Resolving Cell Fate in the Early Embryogenesis of Caenorhabditis elegans
Ruiqi Xiong, Yang Su, Mengchao Yao, Zefei Liu, Jie Lu, Yong-Cong Chen, Ping Ao
Inner ear morphology in wild versus laboratory house micev
Sabrina Renaud, Léa Amar, Pascale Chevret, Caroline Romestaing, Jean-Pierre Quéré, Corinne Régis, Renaud Lebrun
Optimal network sizes for most robust Turing patterns
Hazlam S. Ahmad Shaberi, Aibek Kappassov, Antonio Matas-Gil, Robert G. Endres
Rui Sherry Shen, Yusuf Osmanlıoğlu, Drew Parker, Darien Aunapu, Benjamin E. Yerys, Birkan Tunç, Ragini Verma
Optimizing Non-Intersecting Synthetic Vascular Trees in Nonconvex Organs
Etienne Jessen, Marc C. Steinbach, Dominik Schillinger
Tools & Resources
Unbiased identification of cell identity in dense mixed neural cultures
Sarah De Beuckeleer, Tim Van De Looverbosch, Johanna Van Den Daele, Peter Ponsaerts, Winnok H. De Vos
Daniel Medina-Cano, Mohammed T. Islam, Veronika Petrova, Sanjana Dixit, Zerina Balic, Marty G. Yang, Matthias Stadtfeld, Emily S. Wong, Thomas Vierbuchen
Brian Ho Ching Chan, Holly Hardy, Teresa Requena, Amy Findlay, Jason Ioannidis, Dominique Meunier, Maria Toms, Mariya Moosajee, Anna Raper, Mike McGrew, Joe Rainger
A minimally guided organoid model for cross-species comparisons of cerebellar development
Luca Guglielmi, Daniel Lloyd-Davies-Sánchez, José González Martínez, Madeline A. Lancaster
Enrique Azuaje-Hualde, Naiara Lartitegui-Meneses, Juncal Alonso-Cabrera, Asier Inchaurraga-Llamas, Yara Alvarez-Braña, Marian Martínez de Pancorbo, Fernando Benito-Lopez, Lourdes Basabe-Desmonts
Kirsten Giesbrecht, Simone Rossi, Sophie Liu, Shourya Mukherjee, Michael Bressan, Boyce Griffith
CellMet: Extracting 3D shape metrics from cells and tissues
Sophie Theis, Mario A Mendieta-Serrano, Bernardo Chapa-y-Lazo, Juliet Chen, Timothy E Saunders
CRISPR/Cas9-based somatic knock-in of reporters in the avian embryo in ovo
Alciades Petit Vargas, Baptiste Mida, Rosette Goïame, Olinda Alegria-Prevot, Bojana Djelic, Evelyne Fischer, Samuel Tozer, Jérôme Gros, Marie Manceau, Xavier Morin
Gabriel Bar-Sella, Matan Gavish, Menachem Moshelion
Zebrahub-Multiome: Uncovering Gene Regulatory Network Dynamics During Zebrafish Embryogenesis
Yang Joon Kim, Shruthi Vijay Kumar, Benjamin Iovino, Alejandro Granados, Sarah Ancheta, Xiang Zhao, Kyle Awayan, Amanda Seng, Michael Borja, Sheryl Paul, Honey Mekonen, Ritwicq Arjyal, Angela Detweiler, Yasin Şenbabaoğlu, Rafael Gómez-Sjöberg, Norma Neff, Merlin Lange, Loïc A. Royer
inTRACKtive — A Web-Based Tool for Interactive Cell Tracking Visualization
Teun A.P.M. Huijben, Ashley G. Anderson III, Andrew Sweet, Erin Hoops, Connor Larsen, Kyle Awayan, Jordão Bragantini, Chi-Li Chiu, Loïc A. Royer
Gerard A. Tarulli, Patrick R.S. Tatt, Rhys Howlett, Sara Ord, Beth Shapiro, Stephen R. Frankenberg, Andrew J. Pask
Cole R. McCutcheon, Allyson Caldwell, Liheng (Henry) Yang, Elisa Crisci, J. Alex Pasternak, Carolyn B. Coyne
Generation of human iPSC-derived pancreatic organoids to study pancreas development and disease
Jean-Francois Darrigrand, Abigail Isaacson, Francesca M. Spagnoli
Spatiotemporal map of the developing human reproductive tract at single-cell resolution
Valentina Lorenzi, Cecilia Icoresi Mazzeo, Nadav Yayon, Elias R. Ruiz-Morales, Carmen Sancho-Serra, Frederick C.K. Wong, Magda Marečková, Liz Tuck, Kenny Roberts, Tong Li, Marc-Antoine Jacques, Xiaoling He, Roger Barker, Berta Crespo, Batuhan Cakir, Simon Murray, Martin Prete, Yong Gu, Iva Kelava, Luz Garcia Alonso, John C Marioni, Roser Vento Tormo
Research practice & education
Distinct patterns of bioscience doctoral publication disparities by gender and race/ethnicity
Katie Leap, Gregory S. Payne, Janet S. Sinsheimer, Diana E. Azurdia
Features and signals in precocious citation impact: a meta-research study
John P.A. Ioannidis
Sherri L. Christian, Valerie Booth, Scott Harding, Amy M. Todd, Mark D. Berry
LeDNA: a cut-and-build toolkit to democratize education on CRISPR gene editing technology
Guilherme E. Kundlatsch, Alina S. L. Rodrigues, Vitória F. B. Zocca, Laura A. S. Amorim, Gabriela B. de Paiva, Almiro P. S. Neto, Juliana A. D. B. Campos, Danielle B. Pedrolli
Rui Yip, Young Joo Sun, Alexander G. Bassuk, Vinit B. Mahajan
Quantifying Data Distortion in Bar Graphs in Biological Research
Teng-Jui Lin, Markita P. Landry


 (No Ratings Yet)
(No Ratings Yet) (2 votes)
(2 votes)