In Development this week (Vol. 143, Issue 2)
Posted by Seema Grewal, on 20 January 2016
Here are the highlights from the current issue of Development:
Fishing out a role for Caveolin 1 in heart regeneration

Unlike the adult mammalian heart, the adult zebrafish heart is able to regenerate lost muscle tissue following injury. The epicardial sheet covering the heart is required for this regeneration but the genes that underlie epicardial cell responses to injury are unclear. Here, Jingli Cao and co-workers examine gene expression signatures in zebrafish epicardial cells (p. 232). Using single-cell transcriptome sequencing, the researchers reveal that adult epicardial cells are heterogeneous but exist as at least three main populations, as revealed by hierarchical cluster analyses. The analysis of these populations reveals genes that are expressed in a subset-specific manner as well as pan-epicardial genes, some of which represent novel epicardial markers. The authors further report that caveolin 1 (cav1), which encodes a scaffolding protein that is the main component of caveoli, is expressed pan-epicardially and is required for heart muscle regeneration; although cav1 knockout zebrafish exhibit normal heart development, they display severe defects in injury-induced cardiomyocyte proliferation and heart regeneration. Together, these findings provide key insights into epicardial biology and reveal novel regulators of heart regeneration.
Blimp-1 and PGC specification: of mice and crickets

Germ cells are specified by one of two well-characterised modes: via maternally inherited germ plasm (as seen in the case of Drosophila, C. elegans, Xenopus and zebrafish) or via inductive signals later during embryogenesis (as in the case of many metazoans, including mice). Given that the inductive mode is more prevalent, it has been proposed that it is the ancestral mode of germ cell specification in bilaterians, although molecular evidence for this has been lacking. Now, on p. 255, Taro Nakamura and Cassandra Extavour show that the transcriptional repressor Blimp-1, which is a master regulator of germ cell formation in mice, is required to generate primordial germ cells (PGCs) in the cricket Gryllus bimaculatus. The researchers show that Gb-Blimp-1, the G. bimaculatus homologue of mammalianBlimp-1, is dynamically expressed during germ band elongation. Using RNAi, they further show thatGb-Blimp-1 is required for PGC formation. Finally, the authors demonstrate that, as in mice, Blimp-1 inG. bimaculatus acts downstream of BMP signals to specify cells to a PGC fate. Overall, these findings highlight functional conservation of the relationship between BMP signalling and Blimp-1 during PGC specification, supporting the idea that an inductive mode governed germ cell specification in the last common bilaterian ancestor.
Enteric nervous system development: a role for Shh

Enteric nervous system (ENS) development involves reciprocal interactions between enteric neural crest-derived cells (ENCCs) and their environment as they migrate along the intestine, differentiate, and become patterned. Here, Allan Goldstein, Nandor Nagy and colleagues examine these interactions and reveal that sonic hedgehog (Shh) patterns the extracellular matrix to control enteric nervous system development in chick embryos (p. 264). They report that Shh is expressed specifically in the epithelium of the gut, which harbours an ENS, but not in the epithelium of the bursa of Fabricius, a structure that is associated with the gut but does not have an ENS. They then show, using chick-quail tissue recombinations in which hindgut epithelium is replaced with epithelium from the bursa of Fabricius, that ENS development is perturbed in the absence of hindgut epithelium. Hypothesising that epithelium-derived Shh controls hindgut ENS formation, the authors demonstrate that Shh inhibition causes hyperganglionosis, whereas Shh overexpression causes aganglionosis owing to decreased proliferation and premature differentiation of ENCCs. Finally, they reveal that modulating Shh activity dramatically alters the expression of ECM proteins, such as versican and collagen IX, that are known regulators of neural crest cell migration. These, together with other findings, suggest that epithelial-derived Shh acts indirectly on the developing ENS by regulating the intestinal microenvironment.
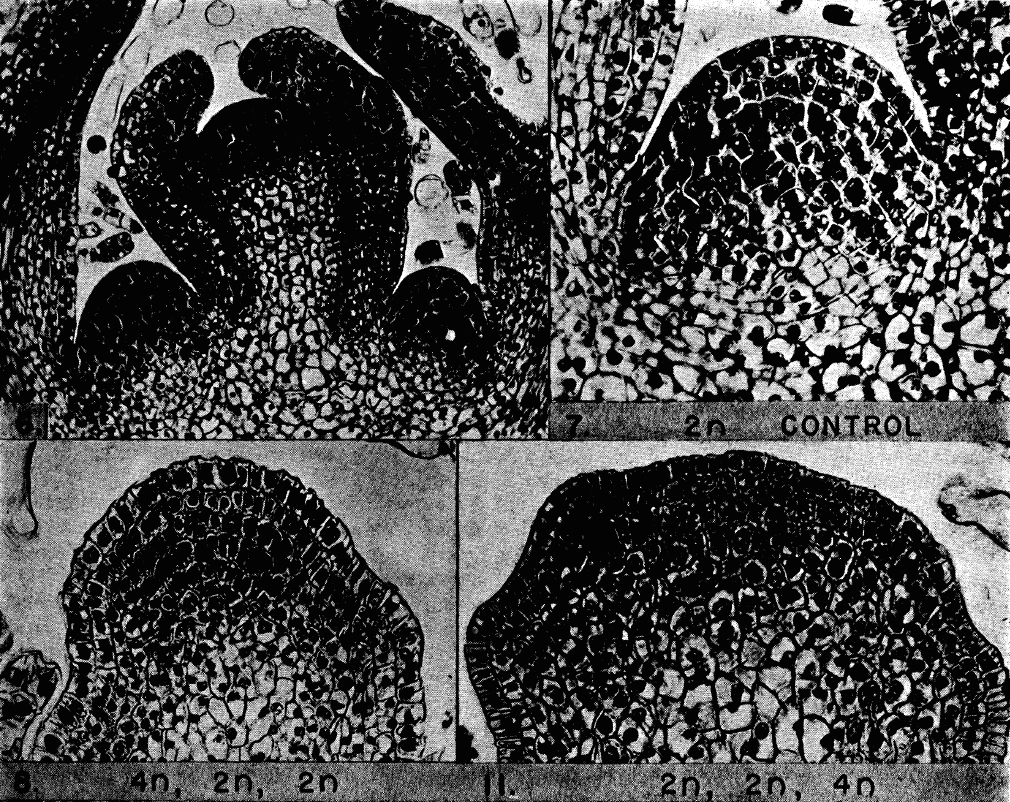
RINGing in PcG function during limb patterning

Polycomb group (PcG) proteins play important roles in regulating gene expression during development but how they contribute to patterning and morphogenetic processes, particularly during mammalian development, is unclear. Here, Haruhiko Koseki and colleagues show that RING1 proteins, which are essential components of Polycomb repressive complex-1 (PRC1), control proximal-distal patterning of the mouse forelimb (p. 276). They demonstrate that Ring1A/B knockout embryos display severe defects in forelimb formation. By analysing gene expression in the distal and proximal regions of the forelimbs in control and mutant animals, they researchers report that RING1 proteins repress the proximal limb regulatory programme in the distal limb bud. Chromatin immunoprecipitation assays reveal that the gene encoding the transcription factor Meis – a known proximal limb bud marker – is bound by RING1 proteins, suggesting that Ring1A/B restrict Meis expression to the proximal limb bud; in line with this, the depletion of Meis2 partially restores distal gene expression and limb formation inRing1-deficient mice. These and other findings lead the authors to propose that PcG factors integrate developmental signals at genes encoding critical transcription factors to regulate patterning during development.
PLUS…
An interview with Peter Lawrence
 Peter Lawrence, FRS, is a fly geneticist based at the Department of Zoology at the University of Cambridge. During his illustrious career he has carried out pioneering work on pattern formation and polarity, and his contributions have been recognised by many honours. He is also an outspoken critic of the current scientific system and particularly how it affects young scientists. We recently had the opportunity to chat with Peter, and we asked him about the influence of his mentor Sir V. B. Wigglesworth, writing his first grant at age 65 and his time as an editor of Development. See the Spotlight article on p. 183
Peter Lawrence, FRS, is a fly geneticist based at the Department of Zoology at the University of Cambridge. During his illustrious career he has carried out pioneering work on pattern formation and polarity, and his contributions have been recognised by many honours. He is also an outspoken critic of the current scientific system and particularly how it affects young scientists. We recently had the opportunity to chat with Peter, and we asked him about the influence of his mentor Sir V. B. Wigglesworth, writing his first grant at age 65 and his time as an editor of Development. See the Spotlight article on p. 183
Measuring forces and stresses in situ in living tissues
 The development, homeostasis and regeneration of tissues result from a complex combination of genetics and mechanics. Sugimora, Lenne and Graner describe techniques to measure forces in cells and tissues, and discuss their applications in developmental contexts. See the Primer on p. 186
The development, homeostasis and regeneration of tissues result from a complex combination of genetics and mechanics. Sugimora, Lenne and Graner describe techniques to measure forces in cells and tissues, and discuss their applications in developmental contexts. See the Primer on p. 186
The formation and function of the cardiac conduction system
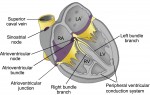 The cardiac conduction system (CCS) consists of distinctive components that initiate and conduct the electrical impulse required for the coordinated contraction of the cardiac chambers. Here, and discuss the complex gene regulatory networks that control the development of the CCS. See the Review article on p. 197
The cardiac conduction system (CCS) consists of distinctive components that initiate and conduct the electrical impulse required for the coordinated contraction of the cardiac chambers. Here, and discuss the complex gene regulatory networks that control the development of the CCS. See the Review article on p. 197
Featured movie
In their latest paper in Development, Martin and colleagues show that the progenitor cells that generate the midline tissues of the zebrafish floor plate, notochord, and hypochord make germ layer decisions after gastrulation based on local canonical Wnt and Notch signaling. This movie is from this paper and shows the posterior of a zebrafish embryo during somitogenesis. The green cells, labelled by injection of fluorescein dextran, were transplanted from another embryo. In the second part of the movie, a cell that will eventually join the notochord is labelled in red. Read their paper here: http://bit.ly/1WasLW


 (No Ratings Yet)
(No Ratings Yet)
 (4 votes)
(4 votes) (17 votes)
(17 votes)

 Marcos and Julia were a brilliant young couple who instantly made the laboratory smart and fun. Marcos took up cancer research, the second person to ever work on this disease in the laboratory. He loved everything about conducting science and he was ambitious. He worked crazy hours. With a laconic Argentinian accent, he kept up a running science conversation that lasted until now. Julia worked on separate topics and they lived balanced lives. Marcos loved board sports from snowboarding to kite- and wind-surfing and was superb at them. Marcos and Julia made friends easily. The Facebook postings and the emails we’ve received and the conversations we’ve had over the past few days are a reminder just how much they were loved. We all rooted for their success because they were really good people.
Marcos and Julia were a brilliant young couple who instantly made the laboratory smart and fun. Marcos took up cancer research, the second person to ever work on this disease in the laboratory. He loved everything about conducting science and he was ambitious. He worked crazy hours. With a laconic Argentinian accent, he kept up a running science conversation that lasted until now. Julia worked on separate topics and they lived balanced lives. Marcos loved board sports from snowboarding to kite- and wind-surfing and was superb at them. Marcos and Julia made friends easily. The Facebook postings and the emails we’ve received and the conversations we’ve had over the past few days are a reminder just how much they were loved. We all rooted for their success because they were really good people.