Senior Post-Doctoral Research Associate in Nanoformulation Safety Assessment using Xenopus laevis
Posted by Grant Wheeler, on 13 February 2015
Closing Date: 15 March 2021
Senior Post-Doctoral Research Associate in Nanoformulation Safety Assessment
(to commence May 4th 2015)
University of East Anglia, Norwich, UK, in collaboration with Procarta Biosystems Ltd, Norwich, UK , Consorzio Interuniversitario Per Lo Sviluppo Dei Sistemi A Grande Interfase (CSGI), Florence, Italy and Nanovector, Turin, Italy, are looking to recruit an experienced researcher. The position will be based at University of East Anglia, for 13 months. The position is funded by the European Union under Framework 7 SP3-PEOPLE program, Support for training and career development of researchers (Marie Curie), “Industry-Academia Partnerships and Pathways” (IAPP), Grant Agreement number 612338, DNA TRAP – Delivery of Nucleic Acid-Based Therapeutics for the Treatment of Antibiotic-Resistant Pathogens.
The recruit should have knowledge and experience of performing safety tests on drugs or nanomaterials using in vitro cell culture models and/or in vivo models such as Xenopus laevis or zebrafish.
The candidate should have a PhD degree in cell biology, molecular biology, nanomedicine or developmental biology or at least 4 years full-time equivalent research experience post-degree. Background in Drug development will be particularly appreciated. Moreover, ability to collaborate in a highly multidisciplinary environment is absolutely required (molecular biologists, chemists, industry and academic partners, materials scientists). The candidate should have excellent interpersonal skills and experience of training or supervising others.
The candidate should not have resided or carried out his/her main activity in the UK for more than 12 months in the 3 years immediately prior to his/her recruitment. Short stays in the UK, such as holidays, are not taken into account. This position offers generous remuneration and includes a monthly mobility allowance, based on the family status of the candidate. Applications are invited from all nationalities, and are not restricted to the European Union countries.
Eligible candidates will be selected based on scientific skill and relevance of their research experience to the project and their ability to meet the criteria regarding mobility and research experience described above. This is an equal opportunity position.
These Marie Skłodowska-Curie Fellowship appointments are offered an annual salary of the Sterling equivalent of €78,624 per annum, plus an additional monthly Mobility Allowance of €1344 for candidates who are married or supporting a dependent child, or €941 without. These amounts are subject to UK employment tax and national insurance, including employer’s national insurance contributions and any other employment costs such as employer pension contributions.
Informal enquiries can be sent to: christopher.j.morris@uea.ac.uk or grant.wheeler@uea.ac.uk
Closing date for applications: 6th March 2015
Proposed interview date: 23rd March 2015


 (No Ratings Yet)
(No Ratings Yet) (3 votes)
(3 votes)
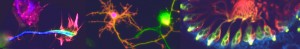

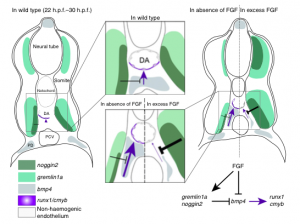
 The dysfunction of hypothalamic neurons is implicated in a number of common diseases, including obesity, hypertension, and mood and sleep disorders. To date, studies of human hypothalamic neurons have been limited due to their inaccessibility, but now (on p.
The dysfunction of hypothalamic neurons is implicated in a number of common diseases, including obesity, hypertension, and mood and sleep disorders. To date, studies of human hypothalamic neurons have been limited due to their inaccessibility, but now (on p.  Directional transport of the plant hormone auxin plays an essential role in plant development. To date, most studies of auxin transport have focussed on the PIN family of auxin efflux transporters but now Jiri Friml and colleagues show that the AUX1 and LIKE-AUX1 (LAX) auxin influx carriers are required during plant embryogenesis (p.
Directional transport of the plant hormone auxin plays an essential role in plant development. To date, most studies of auxin transport have focussed on the PIN family of auxin efflux transporters but now Jiri Friml and colleagues show that the AUX1 and LIKE-AUX1 (LAX) auxin influx carriers are required during plant embryogenesis (p.  Convergent extension (CE) is a morphogenetic process that shapes the early vertebrate embryo. During CE, embryonic tissues elongate along one axis while narrowing in the other, but how are the appropriate forces generated and regulated during this event? Here, Lance Davidson and colleagues investigate the mechanical control of CE in Xenopus embryos (p.
Convergent extension (CE) is a morphogenetic process that shapes the early vertebrate embryo. During CE, embryonic tissues elongate along one axis while narrowing in the other, but how are the appropriate forces generated and regulated during this event? Here, Lance Davidson and colleagues investigate the mechanical control of CE in Xenopus embryos (p.  Over-nutrition and obesity during pregnancy are known to result in lasting developmental and metabolic consequences in offspring. Here, using the Blobby mouse model for obesity, Rebecca Robker and co-workers (p.
Over-nutrition and obesity during pregnancy are known to result in lasting developmental and metabolic consequences in offspring. Here, using the Blobby mouse model for obesity, Rebecca Robker and co-workers (p.  During limb morphogenesis, developing digits are initially interconnected by soft tissue but then become separated as this tissue undergoes programmed cell death (PCD) and regresses. Now, Elazar Zelzer and co-workers demonstrate that vascular patterning in the mouse limb regulates interdigital cell death by a reactive oxygen species (ROS)-dependent mechanism (p.
During limb morphogenesis, developing digits are initially interconnected by soft tissue but then become separated as this tissue undergoes programmed cell death (PCD) and regresses. Now, Elazar Zelzer and co-workers demonstrate that vascular patterning in the mouse limb regulates interdigital cell death by a reactive oxygen species (ROS)-dependent mechanism (p.  Activin/Nodal growth factors control a broad range of biological processes, including early cell fate decisions, organogenesis and adult tissue homeostasis. Here, Siim Pauklin and Ludovic Vallier provide an overview of the mechanisms by which the Activin/Nodal signalling pathway governs stem cell function in these different stages of development. See the Review on p.
Activin/Nodal growth factors control a broad range of biological processes, including early cell fate decisions, organogenesis and adult tissue homeostasis. Here, Siim Pauklin and Ludovic Vallier provide an overview of the mechanisms by which the Activin/Nodal signalling pathway governs stem cell function in these different stages of development. See the Review on p.  Melanocytes have an apparently simple aetiology, differentiating from the neural crest and migrating through the developing embryo to specific locations within the skin and hair follicles, and to other sites in the body. Here, using a cross-species approach, Richard Mort, Ian Jackson and Elizabeth Patton discuss melanocyte development and differentiation, melanocyte stem cells, and the role of the melanocyte lineage in diseases such as melanoma.See the Review on p.
Melanocytes have an apparently simple aetiology, differentiating from the neural crest and migrating through the developing embryo to specific locations within the skin and hair follicles, and to other sites in the body. Here, using a cross-species approach, Richard Mort, Ian Jackson and Elizabeth Patton discuss melanocyte development and differentiation, melanocyte stem cells, and the role of the melanocyte lineage in diseases such as melanoma.See the Review on p.