In Development this week (Vol. 141, Issue 12)
Posted by Seema Grewal, on 10 June 2014
Here are the highlights from the current issue of Development:
Insights into familial dysautonomia
 Familial dysautonomia (FD) is a germline autosomal recessive disease that is characterized by impaired peripheral sensory and sympathetic neuron development. The disease is known to be caused by mutations in the gene encoding Elp1 (also known as IKBKAP), but how Elp1 functions in neurons is unclear. Now, Warren Tourtellotte and colleagues investigate the role of Elp1 in mice (p. 2452). The researchers first generate conditional knockout mice in which Elp1 is ablated from the neural crest progenitors that give rise to sympathetic and sensory neurons. They demonstrate that the loss of Elp1 in these progenitors has no effect on their migration, proliferation, cell fate specification or survival. By contrast, target tissue innervation was perturbed following Elp1 ablation in neural crest progenitors, leading to increased apoptosis of post-migratory sympathetic and sensory neurons. Furthermore, they report that the ablation of Elp1 in post-migratory sympathetic neurons disrupts tissue innervation, and this is associated with attenuated axon branching. In line with this, the authors demonstrate that Elp1-depleted sympathetic and sensory neurons exhibit impaired neurite outgrowth and altered tubulin dynamics, suggesting a role for Elp1 in cytoskeletal regulation. These and future studies of this new mouse model for FD offer promising insights into the role of Elp1 in neural development and disease.
Familial dysautonomia (FD) is a germline autosomal recessive disease that is characterized by impaired peripheral sensory and sympathetic neuron development. The disease is known to be caused by mutations in the gene encoding Elp1 (also known as IKBKAP), but how Elp1 functions in neurons is unclear. Now, Warren Tourtellotte and colleagues investigate the role of Elp1 in mice (p. 2452). The researchers first generate conditional knockout mice in which Elp1 is ablated from the neural crest progenitors that give rise to sympathetic and sensory neurons. They demonstrate that the loss of Elp1 in these progenitors has no effect on their migration, proliferation, cell fate specification or survival. By contrast, target tissue innervation was perturbed following Elp1 ablation in neural crest progenitors, leading to increased apoptosis of post-migratory sympathetic and sensory neurons. Furthermore, they report that the ablation of Elp1 in post-migratory sympathetic neurons disrupts tissue innervation, and this is associated with attenuated axon branching. In line with this, the authors demonstrate that Elp1-depleted sympathetic and sensory neurons exhibit impaired neurite outgrowth and altered tubulin dynamics, suggesting a role for Elp1 in cytoskeletal regulation. These and future studies of this new mouse model for FD offer promising insights into the role of Elp1 in neural development and disease.New atlas-builder software and the eNeuro atlas
 The recent advent of tools for manipulating and monitoring gene expression calls for efficient ways to document, access and analyse these gene expression patterns. Although a number of databases and gene expression atlases have been compiled in recent years, many of them are limited with regards to their content and utility. Here, Chris Doe and colleagues develop new software that overcomes these limitations (p. 2524). This new ‘atlas-builder’ software can be used to create an atlas of gene expression in any tissue in any organism with stereotyped cell positions. Importantly, they report, the atlases generated by this software are three-dimensional, allow for the registration of an infinite number of markers, are searchable and are open-ended; additional markers can be added by users. To validate the software and to help demonstrate its advantages, the authors generated an ‘eNeuro’ atlas of the Drosophilaembryonic CNS. The authors initially populated the atlas with eight transcription factors that mark the major CNS cell types. The atlas was subsequently expanded to include data from 75 Gal4 lines expressed in sparse patterns, thereby allowing the identification of molecularly distinct subsets of interneurons and revealing unexpected diversity among motor neurons. The ‘atlas-builder’ software and the eNeuro atlas, both of which have been made publicly available, promise to be valuable resources for the developmental biology community.
The recent advent of tools for manipulating and monitoring gene expression calls for efficient ways to document, access and analyse these gene expression patterns. Although a number of databases and gene expression atlases have been compiled in recent years, many of them are limited with regards to their content and utility. Here, Chris Doe and colleagues develop new software that overcomes these limitations (p. 2524). This new ‘atlas-builder’ software can be used to create an atlas of gene expression in any tissue in any organism with stereotyped cell positions. Importantly, they report, the atlases generated by this software are three-dimensional, allow for the registration of an infinite number of markers, are searchable and are open-ended; additional markers can be added by users. To validate the software and to help demonstrate its advantages, the authors generated an ‘eNeuro’ atlas of the Drosophilaembryonic CNS. The authors initially populated the atlas with eight transcription factors that mark the major CNS cell types. The atlas was subsequently expanded to include data from 75 Gal4 lines expressed in sparse patterns, thereby allowing the identification of molecularly distinct subsets of interneurons and revealing unexpected diversity among motor neurons. The ‘atlas-builder’ software and the eNeuro atlas, both of which have been made publicly available, promise to be valuable resources for the developmental biology community.
Sp(1)ecifying haematopoietic cells
 Haematopoiesis – the formation of blood cells – is regulated by a number of ubiquitous and tissue-specific transcription factors, but the extent of interplay between these factors is unclear. Sp1 is a transcription factor that is ubiquitously expressed and regulates the expression of thousands of genes, and it has been shown that Sp1-deficient mouse embryos die during early development. Now, on p. 2391, Sjaak Philipsen, Constanze Bonifer and colleagues reveal a crucial role for Sp1 during the early stages of haematopoiesis. Using mouse embryonic stem cells (ESCs) that express a DNA binding-deficient variant of Sp1, the researchers first show that Sp1 activity is required for the differentiation of ESCs to hematopoietic lineages; the cells can progress through most steps of blood cell development but are unable to complete terminal differentiation. Furthermore, they demonstrate that gene expression in Sp1-deficient ESCs becomes progressively deregulated as they differentiate. In particular, they report, some Cdx and Hox family genes that are direct targets of Sp1 are downregulated at an early stage of differentiation, and this is followed by the progressive deregulation of other genes that are implicated in haematopoiesis, suggesting that the effects of Sp1 deficiency are cumulative. Together, these findings identify a crucial role for Sp1 during haematopoiesis and provide detailed insight into the hierarchy of the transcriptional network that drives blood cell formation.
Haematopoiesis – the formation of blood cells – is regulated by a number of ubiquitous and tissue-specific transcription factors, but the extent of interplay between these factors is unclear. Sp1 is a transcription factor that is ubiquitously expressed and regulates the expression of thousands of genes, and it has been shown that Sp1-deficient mouse embryos die during early development. Now, on p. 2391, Sjaak Philipsen, Constanze Bonifer and colleagues reveal a crucial role for Sp1 during the early stages of haematopoiesis. Using mouse embryonic stem cells (ESCs) that express a DNA binding-deficient variant of Sp1, the researchers first show that Sp1 activity is required for the differentiation of ESCs to hematopoietic lineages; the cells can progress through most steps of blood cell development but are unable to complete terminal differentiation. Furthermore, they demonstrate that gene expression in Sp1-deficient ESCs becomes progressively deregulated as they differentiate. In particular, they report, some Cdx and Hox family genes that are direct targets of Sp1 are downregulated at an early stage of differentiation, and this is followed by the progressive deregulation of other genes that are implicated in haematopoiesis, suggesting that the effects of Sp1 deficiency are cumulative. Together, these findings identify a crucial role for Sp1 during haematopoiesis and provide detailed insight into the hierarchy of the transcriptional network that drives blood cell formation.Getting to the root of nodule development
 Root nodulation in plants is a form of de novoorganogenesis and involves the dedifferentiation of root cortical cells in response to rhizobia-derived factors. However, due to the complexity of this event, our understanding of the factors and mechanisms that initiate nodule formation is limited. Now, Takuya Suzaki and co-workers (p. 2441) reveal a role for endoreduplication during the onset of nodule development in Lotus japonicus. The researchers identify novel nodulation-deficient mutants that harbour mutations in VAG1, which encodes a protein that is orthologous to a component of the Arabidopsistopoisomerase VI complex that has been implicated as a regulator of endoreduplication. In line with this, the authors report that the number of endoreduplicated cells in vag1 mutant nodules is reduced compared with that of controls. Importantly, the analysis of nuclear size suggests that VAG1-mediated endoreduplication is crucial for the initiation of nodule formation. Finally, the researchers demonstrate that infection threads, the specialized structures used by rhizobia to invade host cortical cells, elongate towards endoreduplicated cells, and this directional elongation is perturbed in vag1 mutants. These findings highlight an essential role for endoreduplication during root nodule development and suggest that VAG1-mediated endoreduplication is required for the efficient guidance of symbiotic bacteria to host cells.
Root nodulation in plants is a form of de novoorganogenesis and involves the dedifferentiation of root cortical cells in response to rhizobia-derived factors. However, due to the complexity of this event, our understanding of the factors and mechanisms that initiate nodule formation is limited. Now, Takuya Suzaki and co-workers (p. 2441) reveal a role for endoreduplication during the onset of nodule development in Lotus japonicus. The researchers identify novel nodulation-deficient mutants that harbour mutations in VAG1, which encodes a protein that is orthologous to a component of the Arabidopsistopoisomerase VI complex that has been implicated as a regulator of endoreduplication. In line with this, the authors report that the number of endoreduplicated cells in vag1 mutant nodules is reduced compared with that of controls. Importantly, the analysis of nuclear size suggests that VAG1-mediated endoreduplication is crucial for the initiation of nodule formation. Finally, the researchers demonstrate that infection threads, the specialized structures used by rhizobia to invade host cortical cells, elongate towards endoreduplicated cells, and this directional elongation is perturbed in vag1 mutants. These findings highlight an essential role for endoreduplication during root nodule development and suggest that VAG1-mediated endoreduplication is required for the efficient guidance of symbiotic bacteria to host cells.
PLUS…
An interview with Phil Ingham
 Philip Ingham is a geneticist and developmental biologist, based at the Imperial College, London – Nanyang Technological University, Lee Kong Chian School of Medicine in Singapore. Phil has made significant contributions to the developmental biology field over three decades and, in recognition of these achievements, was awarded the Waddington Medal at the 2014 BSDB Spring meeting, where we had the opportunity to interview him. See the Spotlight article on p. 2363
Philip Ingham is a geneticist and developmental biologist, based at the Imperial College, London – Nanyang Technological University, Lee Kong Chian School of Medicine in Singapore. Phil has made significant contributions to the developmental biology field over three decades and, in recognition of these achievements, was awarded the Waddington Medal at the 2014 BSDB Spring meeting, where we had the opportunity to interview him. See the Spotlight article on p. 2363
How to make a hippocampal dentate gyrus granule neuron
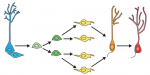 Granule neurons in the hippocampal dentate gyrus (DG) are known to be continuously generated throughout adult life, and the ongoing integration of newborn neurons into the existing hippocampal neural circuitry provides enhanced neuroplasticity, which plays a crucial role in learning and memory. In their Primer article, Gage and colleagues summarize the developmental principles that regulate the process of DG neurogenesis and discuss recent advances in harnessing these developmental cues to generate DG granule neurons from human pluripotent stem cells. See the Primer on p. 2366
Granule neurons in the hippocampal dentate gyrus (DG) are known to be continuously generated throughout adult life, and the ongoing integration of newborn neurons into the existing hippocampal neural circuitry provides enhanced neuroplasticity, which plays a crucial role in learning and memory. In their Primer article, Gage and colleagues summarize the developmental principles that regulate the process of DG neurogenesis and discuss recent advances in harnessing these developmental cues to generate DG granule neurons from human pluripotent stem cells. See the Primer on p. 2366
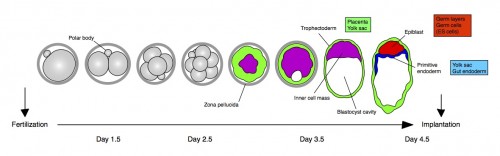
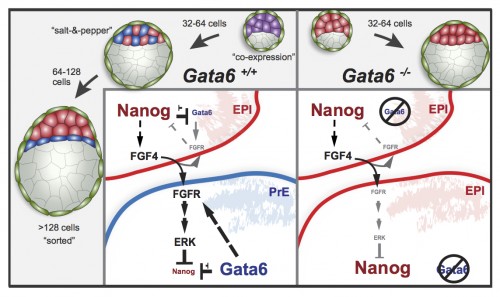
Chromatin features and the epigenetic regulation of pluripotency states in ESCs
 In pluripotent stem cells, the interplay between signaling cues, epigenetic regulators and transcription factors orchestrates developmental potency. Here, Maria-Elena Torres-Padilla and Ian Chambers review what is known about transcriptional heterogeneity in pluripotent stem cells, focusing on the underlying causes of heterogeneity and how transcriptional heterogeneity can be to the benefit of the whole stem cell population. See the Review on p. 2376
In pluripotent stem cells, the interplay between signaling cues, epigenetic regulators and transcription factors orchestrates developmental potency. Here, Maria-Elena Torres-Padilla and Ian Chambers review what is known about transcriptional heterogeneity in pluripotent stem cells, focusing on the underlying causes of heterogeneity and how transcriptional heterogeneity can be to the benefit of the whole stem cell population. See the Review on p. 2376


 (1 votes)
(1 votes)

 (No Ratings Yet)
(No Ratings Yet)

 (5 votes)
(5 votes)