Characterising and visualising cell-type marker genes in Glossina morsitans morsitans.
Posted by Hari Davies, on 27 October 2025
Tsetse flies (Glossina spp.) are the sole vectors of African trypanosomiasis – sleeping sickness in humans and nagana in livestock. The midgut is the site of blood digestion, symbiont interactions, and trypanosome establishment, making it a central organ to study both vector physiology and vector–parasite interactions (Geiger et al. 2013). Understanding the gene expression profiles and cellular organisation underlying midgut function may bring us one step closer to developing new vector control strategies.
During my summer studentship at the Francis Crick Institute, supported by the MRF Rosa Beddington Fund, I worked in Prof. Irene Miguel-Aliaga’s Organ Development and Physiology Laboratory. Mentored by Dr. Mireia Larrosa-Godall, I was able to contribute to ongoing studies of digestive tract physiology in the tsetse fly (Glossina morsitans morsitans).
Understanding organ function requires knowing which cell types are present and how they specialise – diversity that arises not from differences in genetic code but from distinct gene expression patterns. Our lab investigates how gut cells integrate cues, coordinate across tissues, and remodel the organ in response to diet, microbes, sex, or reproduction. While previous research in Drosophila melanogaster has identified different intestinal cell types and their pertinent genetic markers, the cell population of the tsetse fly gut remains unexplored. My project set out to address this gap by characterising candidate intestinal cell marker genes – those showing restricted expression in single-cell RNA-seq clusters, and/or known markers for specific intestinal cell types in D. melanogaster. HCR RNA-FISH was then employed to visualise their spatial mRNA transcript distribution within the gut.
We selected several candidate epithelial marker genes that have well-established roles in D. melanogaster. Enteroendocrine cell fate is specified by the transcription factor gene prospero (Lim et al. 2020), while enterocyte differentiation and microbial tolerance are regulated by nubbin (Widad Dantoft et al. 2013). Maintenance of intestinal stem cell identity is governed by escargot, a canonical intestinal stem cell (ISC) marker (Korzelius et al. 2014), that was not detected in the lab’s single-cell RNA-seq dataset (although this may have been influenced by incomplete 3′ UTR annotation). Beyond the established markers, peptidoglycan recognition protein – pgrp (Bosco-Drayon et al. 2012), involved in the immune pathway, was selected for its cluster-specific expression. In addition, tsetseEP, which encodes a midgut protein with structural similarity to trypanosome proteins and is relevant to vector–parasite interactions (Chandra et al. 2004), and cg12541, a gene of undefined function in D. melanogaster, were also included.
To study the spatial expression of these genes, I started by characterising their mRNA sequence to experimentally confirm transcript isoforms for downstream applications such as probe design. Unlike in other species, the genome assembly available for Glossina morsitans morsitans has fewer predicted genes relative to the more recently available genome assemblies (Attardo et al. 2019). Hence, additional sequencing data would be informative. For this purpose, I designed primers against the predicted sequences and amplified the regions of interest by RT-PCR from Glossina morsitans morsitans cDNA. PCR products were visualised on agarose gels to confirm fragment size, and the amplified DNA was purified. In cases where direct sequencing was not possible, amplicons were cloned by bacterial transformation and screened by colony PCR to identify positive colonies. Verified inserts were then submitted for Sanger sequencing, yielding high-quality reads that were assembled and annotated. In addition to identifying indels specific to the lab’s wild-type strain, four previously unannotated isoforms of prospero were characterised, revealing additional isoform diversity that may regulate enteroendocrine cell specification. The validated sequences (Fig. 1) provided a reliable foundation for the design of HCR probes.
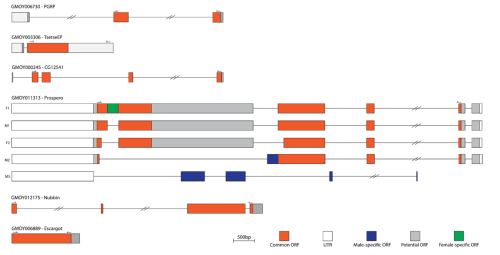
Figure 1. Schematic representation of the transcripts of six candidate intestinal cell marker genes in Glossina morsitans morsitans.
Orange boxes indicate common open reading frames (ORFs), white boxes indicate untranslated regions (UTRs), grey boxes indicate predicted ORFs outside of the sequenced region, blue boxes indicate male-specific ORFs, and the green box indicates a female-specific ORF. Double slashes on introns denote regions longer than 2 kb that are collapsed for clarity. Directional arrows mark sequencing primer positions. Multiple transcript isoforms are shown for prospero (F1, Cn1–2, M1–M2); F = female, M = male, Cn = common. Scale bar represents 500 bp.
In this project, I performed HCR RNA-FISH on two marker genes previously characterised in other insect species – nubbin and escargot – which were expected to label differentiated enterocytes and ISCs, respectively. In this method, whole guts were dissected in PBS and fixed in 4% paraformaldehyde, then incubated with probes complementary to the target mRNA transcripts, along with fluorescent hairpins that enable subsequent chain reaction amplification. Bound probes triggered a chain reaction of fluorescent hairpins that polymerised directly at the mRNA site, producing a strong signal. The tissue was then imaged by confocal microscopy, where the amplified mRNA transcripts appear as bright fluorescent regions within the cell cytoplasm and in some cases within the cell nucleus. This approach offers the first spatial view of mRNA transcription in the tsetse gut.
The nubbin mRNA was detected as nuclear-localised fluorescent signal in a subset of epithelial cells in the middle and posterior midgut (Fig. 2). Its detection in tsetse therefore points to the presence of enterocyte-like cells in these regions. This localisation is consistent with the physiological role of the mid and posterior midgut in nutrient uptake and digestion. By contrast, no nubbin signal was detected in the anterior midgut, consistent with its role as a site of initial blood processing rather than absorption (Wigglesworth, 1929), although this region may contain enterocytes of distinct identity that do not express nubbin.
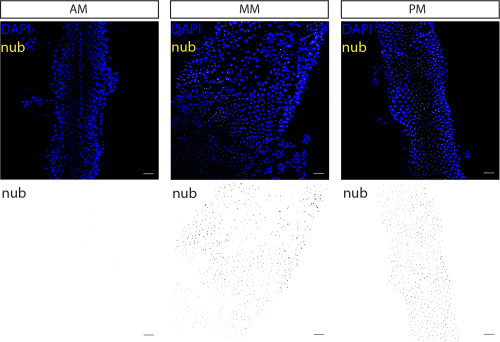
Figure 2. nubbin-expressing cells were identified in the Glossina morsitans morsitans middle midgut and posterior midgut regions.
Confocal z-stacks of female anterior (AM), middle (MM), and posterior midgut (PM) showing nubbin transcripts (yellow) and nuclei counterstained with DAPI (blue). Below, corresponding monochrome panels display nubbin transcript signal in black. Scale bars represent 50 μm for all images. Maximum intensity projections at 20x magnification are shown. Representative image from n = 5.
In contrast, escargot transcripts were not observed in any of the epithelial cells of the midgut (Fig. 3A). Together with their absence from the lab’s single-cell dataset, this supports the possibility that tsetse midguts may lack ISCs. Consistently, pH3 staining – a marker of mitotic activity and therefore used to identify dividing ISCs in the insect gut – did not reveal proliferating cells in the midgut. Positive staining was observed in larval wing discs, suggesting that the absence of pH3 staining in the gut is due to a lack of ISCs (Fig. 3B). Instead, the midgut may depend on alternative mechanisms to maintain gut integrity such as endoreplication, as has been proposed for other blood-feeding insects (Taracena-Agarwal et al. 2024). Nonetheless, it cannot be ruled out that escargot expression occurs at very low levels, within rare populations below detection, or that it is not a marker of ISC identity in the tsetse midgut. Future work will be needed to explore these possibilities.
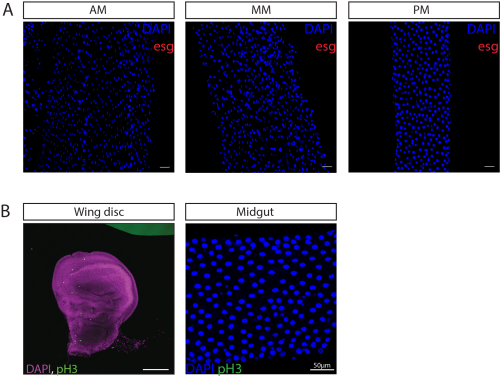
Figure 3. escargot-expressing cells were not identified throughout the Glossina morsitans morsitans midgut regions.
(A) Confocal z-stacks of female anterior (AM), middle (MM), and posterior midgut (PM) showing escargot transcripts (red) and nuclei counterstained with DAPI (blue). Maximum intensity projections at 20× magnification are shown. (B) Immunostaining for phospho-histone H3 (pH3, green) in the larval wing discs (used as a positive control) and adult midgut. Nuclei are counterstained with DAPI (blue in midgut, purple in wing discs). pH3 staining and imaging performed by Lisa Gartner. Scale bars represent 50 μm for all images.
Together, these results provide the first spatial map of nubbin and escargot expression in the tsetse midgut and establish HCR RNA-FISH as a pipeline for visualising gene expression in this species and other blood-feeding insects. This work establishes a foundation for tsetse fly gut physiology, an organ that may influence both reproduction and pathogen interactions, and thus represents a potential target for vector control.
I am very grateful for the opportunity to contribute to research in the Organ Development and Physiology Laboratory at the Francis Crick Institute. Being supported by the MRF Rosa Beddington Fund has been an honour and a formative step in my development as a scientist. This project has been a defining experience, strengthening my commitment to pursue a career in research. I would like to thank the Miguel-Aliaga Lab for their support and for welcoming me into such a stimulating research environment. I am especially grateful to my supervisor, Dr. Mireia Larrosa-Godall, for her guidance and mentorship throughout my project, and to Lisa Gartner for also welcoming me into the fascinating project and contributing the pH3 staining to this study.
Reference list
Attardo, G.M., Abd-Alla, A.M.M., Acosta-Serrano, A., Allen, J.E., Bateta, R., Benoit, J.B., Bourtzis, K., Caers, J., Caljon, G., Christensen, M.B., Farrow, D.W., Friedrich, M., Hua-Van, A., Jennings, E.C., Larkin, D.M., Lawson, D., Lehane, M.J., Lenis, V.P., Lowy-Gallego, E. and Macharia, R.W. (2019). Comparative genomic analysis of six Glossina genomes, vectors of African trypanosomes. Genome Biology, 20(1). doi:https://doi.org/10.1186/s13059-019-1768-2.
Beebe, K., Lee, W.-C. and Micchelli, C.A. (2010). JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Developmental Biology, 338(1), pp.28–37. doi:https://doi.org/10.1016/j.ydbio.2009.10.045.
Bosco-Drayon, V., Poidevin, M., Boneca, I., Narbonne-Reveau, K., Royet, J. and Charroux, B. (2012). Peptidoglycan Sensing by the Receptor PGRP-LE in the Drosophila Gut Induces Immune Responses to Infectious Bacteria and Tolerance to Microbiota. Cell Host & Microbe, 12(2), pp.153–165. doi:https://doi.org/10.1016/j.chom.2012.06.002.
Chandra, M., Liniger, M., Tetley, L., Roditi, I. and Barry, J.D. (2004). TsetseEP, a gut protein from the tsetse Glossina morsitans, is related to a major surface glycoprotein of trypanosomes transmitted by the fly and to the products of a Drosophila gene family. Insect Biochemistry and Molecular Biology, 34(11), pp.1163–1173. doi:https://doi.org/10.1016/j.ibmb.2004.07.004.
Geiger, A., Fardeau, M.-L., Njiokou, F. and Ollivier, B. (2013). Glossina spp. gut bacterial flora and their putative role in fly-hosted trypanosome development. Frontiers in Cellular and Infection Microbiology, 3. doi:https://doi.org/10.3389/fcimb.2013.00034.
Korzelius, J., Naumann, S.K., Loza‐Coll, M.A., Chan, J.S., Dutta, D., Oberheim, J., Gläßer, C., Southall, T.D., Brand, A.H., Jones, D.L. and Edgar, B.A. (2014). Escargot maintains stemness and suppresses differentiation in Drosophila intestinal stem cells. The EMBO Journal, 33(24), pp.2967–2982. doi:https://doi.org/10.15252/embj.201489072.
Lim, S.Y., You, H., Lee, J., Lee, J., Lee, Y., Lee, K.-A., Kim, B., Lee, J.-H., Jeong, J., Jang, S., Kim, B., Choi, H., Hwang, G., Choi, M.S., Yoon, S.-E., Kwon, J.Y., Lee, W.-J., Kim, Y.-J. and Suh, G.S.B. (2020). Identification and characterization of GAL4 drivers that mark distinct cell types and regions in the Drosophila adult gut. Journal of Neurogenetics, 35(1), pp.33–44. doi:https://doi.org/10.1080/01677063.2020.1853722.
Miguel-Aliaga, I., Jasper, H. and Lemaitre, B. (2018). Anatomy and Physiology of the Digestive Tract of Drosophila melanogaster. Genetics, 210(2), pp.357–396. doi:https://doi.org/10.1534/genetics.118.300224.
Taracena-Agarwal, M.L., Hixson, B., S. Nandakumar, Girard-Mejia, A.P., Chen, R.Y., Huot, L., Padilla, N. and N. Buchon (2024). The midgut epithelium of mosquitoes adjusts cell proliferation and endoreplication to respond to physiological challenges. BMC Biology, 22(1). doi:https://doi.org/10.1186/s12915-023-01769-x.
Widad Dantoft, Davis, M.M., Lindvall, J.M., Tang, X., Uvell, H., Junell, A., Beskow, A. and Ylva Engström (2013). The Oct1 homolog Nubbin is a repressor of NF-κB-dependent immune gene expression that increases the tolerance to gut microbiota. BMC Biology, 11(1). doi:https://doi.org/10.1186/1741-7007-11-99.
Wigglesworth, V.B. (1929). Digestion in the Tsetse-Fly: A Study of Structure and Function. Parasitology, 21(3), pp.288–321. doi:https://doi.org/10.1017/s0031182000022988.


 (2 votes)
(2 votes)
 (No Ratings Yet)
(No Ratings Yet)