Here are the highlights from the current issue of Development:
Cofilin and Vangl2 kick start planar cell polarity
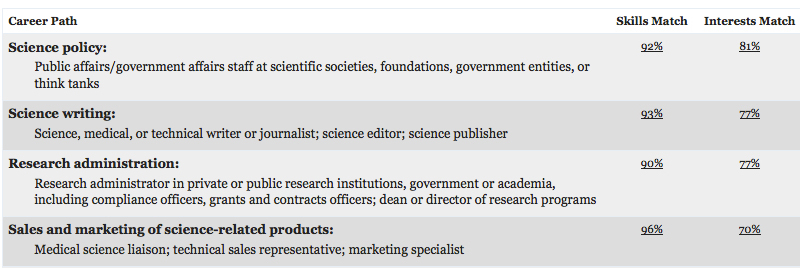
 The planar cell polarity (PCP) pathway orients cells within the plane of an epithelium during development. Experiments in Drosophila indicate that the core PCP proteins move to the apical cell membrane during the initiation of PCP and implicate the actin-severing protein cofilin in PCP initiation. Now, on p. 1262, Kathyrn Anderson and colleagues report that cofilin 1 (Cfl1) and the core PCP protein Vangl2 cooperate to control PCP initiation in the mouse embryo. The researchers analyse two aspects of PCP – convergent extension of the axial midline and posterior positioning of nodal cilia. Both these aspects of PCP are nearly normal in Cfl1 and Vangl2 single mutants, they report, but midline extension fails completely and nodal cilia do not polarise in Vangl2 Cfl1 double mutants because PCP protein complexes fail to move to the apical cell membrane. These and other results suggest that remodelling of the actin cytoskeleton is required to traffic vesicles containing PCP proteins to the apical membrane during PCP initiation.
The planar cell polarity (PCP) pathway orients cells within the plane of an epithelium during development. Experiments in Drosophila indicate that the core PCP proteins move to the apical cell membrane during the initiation of PCP and implicate the actin-severing protein cofilin in PCP initiation. Now, on p. 1262, Kathyrn Anderson and colleagues report that cofilin 1 (Cfl1) and the core PCP protein Vangl2 cooperate to control PCP initiation in the mouse embryo. The researchers analyse two aspects of PCP – convergent extension of the axial midline and posterior positioning of nodal cilia. Both these aspects of PCP are nearly normal in Cfl1 and Vangl2 single mutants, they report, but midline extension fails completely and nodal cilia do not polarise in Vangl2 Cfl1 double mutants because PCP protein complexes fail to move to the apical cell membrane. These and other results suggest that remodelling of the actin cytoskeleton is required to traffic vesicles containing PCP proteins to the apical membrane during PCP initiation.
Chromatin remodelling in vein specification
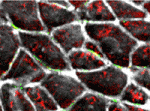
 Arteries and veins are structurally and functionally distinct vessels that circulate blood away from and towards the heart, respectively. Notch signalling determines arterial specification during development whereas the orphan nuclear receptor COUP-TFII (also known as NR2F2) promotes venous specification by inhibiting Notch signalling in a subset of endothelial cells. But what regulates COUP-TFII expression in veins? Courtney Griffin and co-workers now report (p. 1272) that the chromatin remodelling enzyme BRG1 promotes COUP-TFII expression and venous specification during mouse embryogenesis. The researchers show that genetic depletion of Brg1 downregulates COUP-TFII expression and leads to aberrant expression of arterial markers in developing veins. BRG1 promotes the expression of COUP-TFII, they report, by binding to regulatory elements within the COUP-TFII promoter and remodelling the chromatin to increase the promoter’s accessibility to the transcriptional machinery. These data describe for the first time a factor that promotes COUP-TFII expression in developing veins and broaden our understanding of how epigenetic processes influence vascular development.
Arteries and veins are structurally and functionally distinct vessels that circulate blood away from and towards the heart, respectively. Notch signalling determines arterial specification during development whereas the orphan nuclear receptor COUP-TFII (also known as NR2F2) promotes venous specification by inhibiting Notch signalling in a subset of endothelial cells. But what regulates COUP-TFII expression in veins? Courtney Griffin and co-workers now report (p. 1272) that the chromatin remodelling enzyme BRG1 promotes COUP-TFII expression and venous specification during mouse embryogenesis. The researchers show that genetic depletion of Brg1 downregulates COUP-TFII expression and leads to aberrant expression of arterial markers in developing veins. BRG1 promotes the expression of COUP-TFII, they report, by binding to regulatory elements within the COUP-TFII promoter and remodelling the chromatin to increase the promoter’s accessibility to the transcriptional machinery. These data describe for the first time a factor that promotes COUP-TFII expression in developing veins and broaden our understanding of how epigenetic processes influence vascular development.
Airn silencing: self-sufficient but reinforced
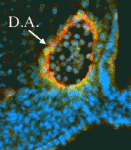
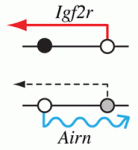 Epigenetic processes control the parental-specific (imprinted) expression of a subset of mammalian genes. For example, the paternally expressed imprinted long non-coding (lnc) RNA Airn initiates paternal-specific silencing of Igf2r, a gene that is essential for development. Airn initiation of Igf2r silencing is followed by gain of DNA methylation on the silent Igf2r promoter. Here (p. 1184), Denise Barlow, Florian Pauler and colleagues investigate the control of Igf2r silencing during mouse embryonic stem cell (ESC) differentiation. By turning Airn expression off during ESC differentiation, the researchers show that continuous Airn expression is needed to maintain Igf2r silencing until the paternal Igf2r promoter is methylated. By conditionally turning Airn expression on, they show that Airn can initiate Igf2r silencing throughout ESC differentiation and that silencing is maintained in the absence of DNA methylation. Thus, Airn lncRNA is necessary and sufficient to silence Igf2r throughout ESC development whereas DNA methylation is dispensable for silencing initiation and maintenance but reinforces Igf2r silencing.
Epigenetic processes control the parental-specific (imprinted) expression of a subset of mammalian genes. For example, the paternally expressed imprinted long non-coding (lnc) RNA Airn initiates paternal-specific silencing of Igf2r, a gene that is essential for development. Airn initiation of Igf2r silencing is followed by gain of DNA methylation on the silent Igf2r promoter. Here (p. 1184), Denise Barlow, Florian Pauler and colleagues investigate the control of Igf2r silencing during mouse embryonic stem cell (ESC) differentiation. By turning Airn expression off during ESC differentiation, the researchers show that continuous Airn expression is needed to maintain Igf2r silencing until the paternal Igf2r promoter is methylated. By conditionally turning Airn expression on, they show that Airn can initiate Igf2r silencing throughout ESC differentiation and that silencing is maintained in the absence of DNA methylation. Thus, Airn lncRNA is necessary and sufficient to silence Igf2r throughout ESC development whereas DNA methylation is dispensable for silencing initiation and maintenance but reinforces Igf2r silencing.
T cell to myeloid cell switch
 T cells develop from multipotent progenitors in the thymus. Initially, these progenitors can generate myeloid cells, B lymphocytes and T cells but, as differentiation proceeds, they become committed to the T-cell lineage. On p. 1207, Marissa Morales Del Real and Ellen Rothenberg investigate the regulatory network that controls this process. Previous studies have shown that the decision to become a T cell can be opposed by the myeloid cell transcription factor PU.1 but that exposure to Notch signalling determines the developmental outcome of expressing PU.1. The researchers now show that Notch signalling does not inactivate the PU.1 protein but instead re-channels its transcriptional effects to maintain a T-cell transcriptional network. They describe two branches of this network – one that involves basic helix-loop-helix E proteins in a positive-feedback loop with Notch, and one in which PU.1 can inhibit T-cell transcription factor genes such as Gata3 only if Notch signalling is absent. Together, these results provide new insights into the complex architecture of a lymphomyeloid developmental switch.
T cells develop from multipotent progenitors in the thymus. Initially, these progenitors can generate myeloid cells, B lymphocytes and T cells but, as differentiation proceeds, they become committed to the T-cell lineage. On p. 1207, Marissa Morales Del Real and Ellen Rothenberg investigate the regulatory network that controls this process. Previous studies have shown that the decision to become a T cell can be opposed by the myeloid cell transcription factor PU.1 but that exposure to Notch signalling determines the developmental outcome of expressing PU.1. The researchers now show that Notch signalling does not inactivate the PU.1 protein but instead re-channels its transcriptional effects to maintain a T-cell transcriptional network. They describe two branches of this network – one that involves basic helix-loop-helix E proteins in a positive-feedback loop with Notch, and one in which PU.1 can inhibit T-cell transcription factor genes such as Gata3 only if Notch signalling is absent. Together, these results provide new insights into the complex architecture of a lymphomyeloid developmental switch.
Hear, hear! Postnatal cochlear cell progenitors
 Irreversible damage of cochlear sensory hair cells and nonsensory supporting cells causes permanent hearing loss because the sensory epithelium cannot repair or regenerate itself postnatally. Active Wnt/β-catenin signalling marks many endogenous stem cells and Roel Nusse, Alan Gi-Lun Cheng and colleagues now report (p.1196) that tympanic border cells (TBCs), which lie beneath the sensory epithelium, are Wnt responsive and can act as progenitors for sensory epithelial cells in the postnatal mouse cochlea. The researchers show that transient but robust Wnt signalling and proliferation exists in TBCs during the first 3 postnatal weeks and report that Wnt agonists stimulate the proliferation of TBCs in cochlear explants. Moreover, TBCs that express the Wnt target gene Axin2 can generate new hair cells and supporting cells in vivo and in vitro. The researchers suggest, therefore, that TBCs serve as a reservoir of cells for the intricate organisation of the cochlea during early postnatal development and that quiescent TBCs in the adult cochlea might represent targets for regenerative therapy.
Irreversible damage of cochlear sensory hair cells and nonsensory supporting cells causes permanent hearing loss because the sensory epithelium cannot repair or regenerate itself postnatally. Active Wnt/β-catenin signalling marks many endogenous stem cells and Roel Nusse, Alan Gi-Lun Cheng and colleagues now report (p.1196) that tympanic border cells (TBCs), which lie beneath the sensory epithelium, are Wnt responsive and can act as progenitors for sensory epithelial cells in the postnatal mouse cochlea. The researchers show that transient but robust Wnt signalling and proliferation exists in TBCs during the first 3 postnatal weeks and report that Wnt agonists stimulate the proliferation of TBCs in cochlear explants. Moreover, TBCs that express the Wnt target gene Axin2 can generate new hair cells and supporting cells in vivo and in vitro. The researchers suggest, therefore, that TBCs serve as a reservoir of cells for the intricate organisation of the cochlea during early postnatal development and that quiescent TBCs in the adult cochlea might represent targets for regenerative therapy.
Tumour suppression trafficked by Atg6
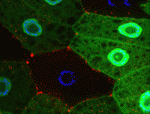 Autophagy is a conserved catabolic process that degrades the cell’s own components through the lysosomal machinery in response to cell stress. Atg6/beclin 1 is a core component of the mammalian vacuolar protein sorting 34 (Vps34) complex that is required for autophagy. It is also a tumour suppressor, a function that has been attributed to its role in autophagy. But could the potential function of Atg6/beclin 1 in other vesicle trafficking pathways be involved in tumour development? On p. 1321, Eric Baehrecke and co-workers generate Atg6 mutant Drosophila and show that Atg6 is essential for autophagy, endocytosis and protein secretion. By contrast, the core autophagy gene Atg1 is required for autophagy and protein secretion only. Consistent with the tumour suppressor role of beclin 1, loss of Atg6 causes over-production of blood cells and the formation of melanotic blood cell masses. Together, these results suggest that the involvement of Atg6/beclin 1 in multiple vesicle trafficking pathways underlies its role as a tumour suppressor.
Autophagy is a conserved catabolic process that degrades the cell’s own components through the lysosomal machinery in response to cell stress. Atg6/beclin 1 is a core component of the mammalian vacuolar protein sorting 34 (Vps34) complex that is required for autophagy. It is also a tumour suppressor, a function that has been attributed to its role in autophagy. But could the potential function of Atg6/beclin 1 in other vesicle trafficking pathways be involved in tumour development? On p. 1321, Eric Baehrecke and co-workers generate Atg6 mutant Drosophila and show that Atg6 is essential for autophagy, endocytosis and protein secretion. By contrast, the core autophagy gene Atg1 is required for autophagy and protein secretion only. Consistent with the tumour suppressor role of beclin 1, loss of Atg6 causes over-production of blood cells and the formation of melanotic blood cell masses. Together, these results suggest that the involvement of Atg6/beclin 1 in multiple vesicle trafficking pathways underlies its role as a tumour suppressor.
PLUS…
Epigenetic mechanisms in the development and maintenance of dopaminergic neurons
Marten Smidt and colleagues review the epigenetic control of mdDA development, maturation and maintenance. See the Review article on p. 1159
Gibberellin signaling in plants
The plant hormone gibberellin (GA) regulates major aspects of plant growth and development. Jean-Michel Daviere and Patrick Achard review the molecular basis of the GA signaling pathway, from the perception of GA to the regulation of downstream genes. See the Development at a Glance poster article on p. 1147
Auxin 2012: a rich mea ho’oulu
In December 2012, scientists from around the world gathered in Waikoloa, Hawaii for ‘Auxin 2012’. At the meeting, participants discussed the latest advances in auxin biosynthesis, transport and signaling research, in addition to providing context for how these pathways intersect with other aspects of plant physiology and development. See the Meeting Review on p. 1153
 (No Ratings Yet)
(No Ratings Yet)
 Loading...
Loading...


 (1 votes)
(1 votes)

 Jessica Whited works with a completely different animal model. She recently developed a
Jessica Whited works with a completely different animal model. She recently developed a  The planar cell polarity (PCP) pathway orients cells within the plane of an epithelium during development. Experiments in Drosophila indicate that the core PCP proteins move to the apical cell membrane during the initiation of PCP and implicate the actin-severing protein cofilin in PCP initiation. Now, on
The planar cell polarity (PCP) pathway orients cells within the plane of an epithelium during development. Experiments in Drosophila indicate that the core PCP proteins move to the apical cell membrane during the initiation of PCP and implicate the actin-severing protein cofilin in PCP initiation. Now, on  Arteries and veins are structurally and functionally distinct vessels that circulate blood away from and towards the heart, respectively. Notch signalling determines arterial specification during development whereas the orphan nuclear receptor COUP-TFII (also known as NR2F2) promotes venous specification by inhibiting Notch signalling in a subset of endothelial cells. But what regulates COUP-TFII expression in veins? Courtney Griffin and co-workers now report (
Arteries and veins are structurally and functionally distinct vessels that circulate blood away from and towards the heart, respectively. Notch signalling determines arterial specification during development whereas the orphan nuclear receptor COUP-TFII (also known as NR2F2) promotes venous specification by inhibiting Notch signalling in a subset of endothelial cells. But what regulates COUP-TFII expression in veins? Courtney Griffin and co-workers now report ( Epigenetic processes control the parental-specific (imprinted) expression of a subset of mammalian genes. For example, the paternally expressed imprinted long non-coding (lnc) RNA Airn initiates paternal-specific silencing of Igf2r, a gene that is essential for development. Airn initiation of Igf2r silencing is followed by gain of DNA methylation on the silent Igf2r promoter. Here (
Epigenetic processes control the parental-specific (imprinted) expression of a subset of mammalian genes. For example, the paternally expressed imprinted long non-coding (lnc) RNA Airn initiates paternal-specific silencing of Igf2r, a gene that is essential for development. Airn initiation of Igf2r silencing is followed by gain of DNA methylation on the silent Igf2r promoter. Here ( T cells develop from multipotent progenitors in the thymus. Initially, these progenitors can generate myeloid cells, B lymphocytes and T cells but, as differentiation proceeds, they become committed to the T-cell lineage. On
T cells develop from multipotent progenitors in the thymus. Initially, these progenitors can generate myeloid cells, B lymphocytes and T cells but, as differentiation proceeds, they become committed to the T-cell lineage. On  Irreversible damage of cochlear sensory hair cells and nonsensory supporting cells causes permanent hearing loss because the sensory epithelium cannot repair or regenerate itself postnatally. Active Wnt/β-catenin signalling marks many endogenous stem cells and Roel Nusse, Alan Gi-Lun Cheng and colleagues now report (
Irreversible damage of cochlear sensory hair cells and nonsensory supporting cells causes permanent hearing loss because the sensory epithelium cannot repair or regenerate itself postnatally. Active Wnt/β-catenin signalling marks many endogenous stem cells and Roel Nusse, Alan Gi-Lun Cheng and colleagues now report ( Autophagy is a conserved catabolic process that degrades the cell’s own components through the lysosomal machinery in response to cell stress. Atg6/beclin 1 is a core component of the mammalian vacuolar protein sorting 34 (Vps34) complex that is required for autophagy. It is also a tumour suppressor, a function that has been attributed to its role in autophagy. But could the potential function of Atg6/beclin 1 in other vesicle trafficking pathways be involved in tumour development? On
Autophagy is a conserved catabolic process that degrades the cell’s own components through the lysosomal machinery in response to cell stress. Atg6/beclin 1 is a core component of the mammalian vacuolar protein sorting 34 (Vps34) complex that is required for autophagy. It is also a tumour suppressor, a function that has been attributed to its role in autophagy. But could the potential function of Atg6/beclin 1 in other vesicle trafficking pathways be involved in tumour development? On  (No Ratings Yet)
(No Ratings Yet)