What would you do if you were given $500,000 to fund your research for five years, with no strings attached –– no proposals to write, no progress reports to submit? If you were one of the recently announced recipients of the prestigious MacArthur fellowship you would be giving this question some serious thought.
What would you do if you were given $500,000 to fund your research for five years, with no strings attached –– no proposals to write, no progress reports to submit? If you were one of the recently announced recipients of the prestigious MacArthur fellowship you would be giving this question some serious thought.
Every year since 1981 the John D. and Catherine T. MacArthur Foundation recognizes exceptionally creative individuals by awarding them with $500,000 “genius grants”. According to the press release published on September 20th “MacArthur fellowships come without stipulations or reporting requirements and offer fellows unprecedented freedom and opportunity to reflect, create, and explore. All [fellows] were selected for their creativity, originality, and potential to make important contributions in the future.”
To be considered for the MacArthur fellowship requires a nomination. However the identities of the nominators as well as the selection process are kept in complete secrecy. Those who are selected for the award find out through an “out of the blue” phone call from the foundation two weeks prior to the official announcement. This year’s 22 recipients include a musician, a poet, a historian, an economist, a radio producer as well as 10 scientists. Among them is Yukiko Yamashita a developmental biologist and assistant research professor at the University of Michigan who studies the mechanisms regulating stem cell division in the context of unperturbed tissue anatomy –– adult testes.
Getting the Call
When Yukiko received the phone call informing her about being selected and asking her asking her not to discuss it with anyone except her spouse until the official announcement, she had a hard time believing that it was not a scam. “I called my husband right after I hung up my phone call with the foundation and [he] seriously warned me that ‘if you get a second phone call asking your bank account and pin number, so that they can transfer the award money, don’t give it to them.’”
They did not ask for her bank info but did want to bring a production crew to her lab to film an interview for the foundation’s website. “I had to ask the director of my institute if the production crew can come into the building to film me before the press release. We seriously have to worry about some activists against research blowing up the building. I didn’t want to be the stupid assistant professor who believed they are awarded a MacArthur ended up with destroying the entire institute.” When the names of this years MacArthur fellows were finally announced publicly Yukiko felt more relieved than ecstatic.
Yukiko completed her undergraduate and doctoral studies in Japan, at Kyoto University, followed by postdoctoral training with Margaret Fuller in the Department of Developmental Biology at Stanford University. In 2007 she established her own lab as an assistant research professor at the University of Michigan Medical School.
Choose Your Centrosome Wisely
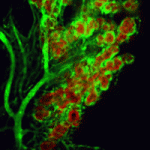
Her current research, which has won her the recognition from the MacArthur foundation, combines cell and developmental biology and is focused on investigating molecular and cellular mechanisms governing stem cell behavior, using the Drosophila male germline stem cells (GSCs) as a model. Her lab is investigating how stem cell division is regulated and how different stem cell populations interact to maintain tissue stability.
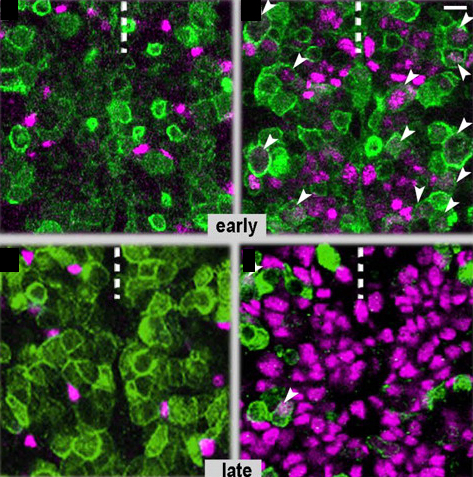
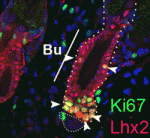
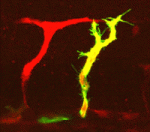
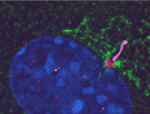
During her time at Stanford, Yukiko made the discovery that the centrosomes of Drosophila male GSCs are non-randomly segregated during asymmetric division –– the older “mother” centrosome remains with the stem cell while the newly replicated “daughter” centrosome is inherited by the differentiating cell.
The GSCs in Drosophila reside in a niche composed somatic support cells called hub cells and cyst stem cells. GSCs attach to the support cells at their apical side and during cell division orient the mitotic spindle along the apical-basal axis. The cells still attached to the hub after cell division maintain a stem fate, while the daughter cell, displaced from the hub, differentiates.
“Its been known for quite a long time in the cell biology field that mother and daughter centrosomes are a little different from each other. The newborn centriole in the centrosome takes more than one cell cycle to get fully mature,” says Yukiko. As the centriole matures it accumulates structures called subdistal appendages, which serve to anchor the microtubles forming the spindle. Mature centrosomes therefore have a stronger ability to anchor microtubules. “Because of this difference the mother centrosome always has higher microtubule nucleating capacity.” In fact Yukiko’s found that the mother centrosome is anchored by microtubules at the apical pole of the cell, near the hub. “That is why, we are guessing, the mother centrosome can stay close to the hub cells all the time.”
However this mechanism is not universal to all stem cells. “In Drosophila neuroblasts, every single cell cycle the mother centrosome gets inactivated and the daughter centromosme gets activated and so, unconventionally, the daughter centrosome has higher [microtubule organizing center] MTOC activity. In the end the stem cell ends up inheriting the daughter centrosome all the time.” The reasoning for this switching is not known, however the trend is that the centrosome with higher MTOC activity is inherited by the stem cell. “In one case, germ line stem cells maintain higher MTOC activity on the mother centrosome, but in the Drosophila neuroblasts they make daughter centrosome with high MTOC activity. Why this is happening we don’t know. But once you have high MTOC activity it looks like that’s going to the stem cell.”
How Do Chromosomes Fit Into the Picture?
Following Yukiko’s discovery about the non-random segregation of centrosomes other scientists in the field speculated that it might serve as a mechanism to selectively segregate chromosomes, perhaps keeping the original strands in the stem cell as proposed by the immortal strand hypothesis. Yukiko, however, was not convinced that this was the case and thought that a thorough analysis was required to prove or disprove the hypothesis –– something that she felt was lacking in some studies.
“We thought we really should address this question in our system, in which we can directly test this idea,” she says. In early 2011 Yukiko’s group published a paper in the Journal of Cell Science reporting that the chromosomes of GSCs, unlike the centrosomes, are randomly segregated. “I’m glad that we published this paper because I think [among] the immortal strand hypothesis papers, some are really good and the data appears really convincing, but some others are not really excluding alternative possibilities or different interpretations. I really wanted to propose some rigorous way of testing it. I’m not saying that the immortal strand [hypothesis] is not correct, but then to make it right, you have to examine every single possibility.”
Since that publication, a the graduate student pursuing this line of research in her lab has examined the segregation of each individual strand for all the chromosomes and found that at this level of resolution only a small subset of chromosomes are selectively segregated, while the segregation of others is random. “It looks like germline stem cells are segregating very specific strands with quite high bias only for some of the chromosomes, but not others, so that at least suggests that cells have the machinery to distinguish one chromosome strand over the other and then segregate one into the stem cell in a biased way.”
This finding that the majority of the chromosomes are randomly segregated still leaves the question of why cells need to selectively segregate their centrosomes? “Ultimately the question everybody is asking is: does the mother or daughter centrosome carry some information, not just microtubules?”
Yukiko doesn’t yet have the answer but can speculate about the possibilities. “The centrosome itself [could be] associated with some sort of fate determinants. That is not unprecedented. Some fate determining mRNA is associated with just one centrosome during mollusk early embryogenesis. [Another possibility is that] the centrosomes are used to distinguish two different sister chromatids, to segregate one strand over the other. Why you have to distinguish one strand over the other strand of the chromosome? I don’t think its for the sake of an immortal strand, I don’t think its because of the DNA mutations or avoiding them, instead I think it’s some epigenetic information that they want to carry. It might be histone modifications or DNA methylation but we don’t have any evidence for that yet.”
Checks and Balances
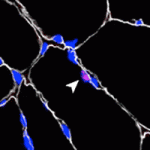
In addition to their work on centrosome segregation Yukiko’s group is pursuing two other lines of research. One is examining a novel cell cycle checkpoint, which ensures the correct orientation of centrosomes prior to cell division. “We published one paper suggesting the presence of a new checkpoint that, in GSCs, makes sure the centrosomes are correctly oriented before they get into mitosis. If the centrosomes are not oriented correctly, this checkpoint gets activated and then stalls the cell cycle before entering mitosis. We really want to identify the mechanism of how stem cells are sensing the correct orientation of the centrosome before getting into mitosis.”
The idea of a cell-cycle checkpoint is more at home with cell biologists and Yukiko thinks that it will take some time to convince developmental biologists that what they are describing is a real phenomenon. “I think its going to be a long way to really prove that this really exists, exactly how cells are sensing it, and what is the molecular mechanism, etc. It will probably take multiple papers and probably quite long time; at least five years if not 10 years.”
Yukiko also wants to understand how different stem cell populations interact in tissues and communicate to coordinate their replication and life cycles. This is a new line of research for the lab and Yukiko wants to explore this direction in the coming years.
“[The Drosophila male gonadal] stem cell niche contains yet another type of stem cell called cyst stem cell. The germline stem cells and cyst stem cells have to coordinate their divisions somehow, we don’t know exactly how yet. Many tissues are made of cell types that are coming from different stem cell lineages. That means the decision of one single stem cell population cannot be enough to maintain the whole tissue. One stem cell population has to coordinate with another stem cell population to make sure that tissues are maintained as a whole. I’m very, very interested in how the stem cells are coordinating with each other.”
Follow Your Passion
What does getting the MacArthur fellowship mean for the future directions in the lab? Most importantly in means freedom to pursue any interesting outcomes that arise in research without the constraints of sticking to a proposed research plan. “The whole idea of being a scientist is that you can’t really predict anything. If you’re working on something and the answer is so predictable, its quite boring. Now I feel I do have the freedom to wander off a little bit from the original plan, because of course I didn’t propose anything for the MacArthur!”
Having a passion for science it vital for success as a scientist, but it doesn’t mean that your whole life has to be about work. Yukiko admits that she used to get worried when life’s distractions took too much time away from the bench. What helped her to establish a career as an independent researcher and develop a life-work balance was learning “laid-back confidence” from her postdoc mentor Margaret Fuller. “She’s a really good scientist but she is not obsessed by success. You can love science, but it doesn’t have to be your whole life. Other things enter your life that may take time away from the science, but don’t worry. It might take you a little longer, but you will get to the point where you want to go if you just continue what you’re doing. That is something I learned from her.”
Yukiko spends her free time with her family and taking care of her six-year old daughter. I asked Yukiko if there is something people would be surprised to learn about her. “I am really obsessed with fossil hunting,” she said. In Michigan, which used to be under tropical water millions of years ago, finding fossilized coral can be as easy as examining the pebbles on the road for a few minutes. “It’s becoming a fun hobby for my daughter and me.” After some thought she added: “And another thing my husband always teases me about ‘Where is this assistant professor who sleeps 8-9 hours a day?’ It’s how much I sleep every day!”
For more information:
Profile of Yukiko Yamashita on the MacArthur Foundation website
Press release from the MacArthur Foundation announcing this year’s fellows
Yukiko Yamashita’s lab webpage
References
Yadlapalli Swathi, ChengJun, YamashitaYukiko M. (2011). Drosophila male germline stem cells do not asymmetrically segregate chromosome strands. Journal of Cell Science, 124, 933-9.
Yamashita Yukiko M. (2010). A tale of mother and daughter. Molecular biology of the cell, 21, 7-8.
Yamashita Yukiko M. (2009). The centrosome and asymmetric cell division. Prion, 3, 84-8.
 (4 votes)
(4 votes)
 Loading...
Loading...


 (No Ratings Yet)
(No Ratings Yet) When did you first become interested in plant development?
When did you first become interested in plant development?

 Who or what inspired you to study science?
Who or what inspired you to study science? (2 votes)
(2 votes)