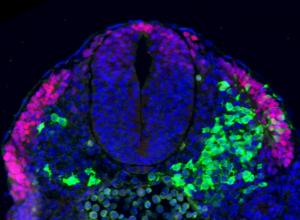
Researchers generate for the first time Drosophila melanogaster with intestinal cancer and reveal key genetic factors behind human colon cancer.
The scientists identify a human gene that favours the proliferation of tumour cells in early stages of colon cancer.
Furthermore, the flies are useful for faster and more economic drug screening.
Researchers at the Institute for Research in Biomedicine (IRB Barcelona) have managed to generate a fruit fly (Drosophila melanogaster) model that reproduces human colon cancer. With two publications appearing in PLoS One and EMBO Reports, the IRB team also unveil the function of a key gene in the development of the disease.
“The breakthrough is that we have generated cancer in an adult organism and from stem cells, thus reproducing what happens in most types of human cancer. This model has allowed us to identify subtle interactions in the development of cancer that are practically impossible to detect in mice with the current technology available,” explains the biologist Andreu Casali, Associate Researcher at IRB Barcelona and leader of the Drosophila project.
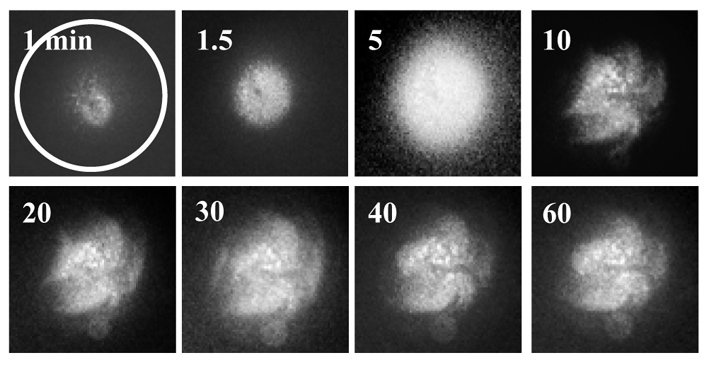
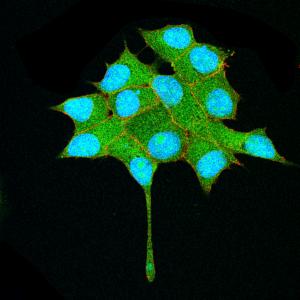
Although the flies do not have a colon, they have an intestine that includes a colon and rectum and that works in the same way as the human colon. The scientists generated mutant flies for two genes that are altered in most human colon tumours, namely APC and Ras. Thanks to the ease with which genetic studies can be performed in Drosophila, the researchers were able to examine the effect of 250 genes that are altered in these types of tumour and found that, of these, 30% affected growth while the others had no significant effects.
“The advantage of the model is that it allows us to explore genetic alterations more quickly, to distinguish between those that are important and those that are not, and to see what role they play,” explains Òscar Martorell, first author of the paper that appears in EMBO Reports published today. “Performing these genetic experiments in mice is time-consuming and costly and the Drosophila model allows us to rapidly analyse new pathways that could be relevant for colon cancer,” adds the co-author of the study, Francisco Barriga, a postdoctoral fellow working on colon cancer in vertebrate models. Undertaken over five years, the study is the result of collaboration between the Development and Morphogenesis in Drosophila Lab and the Colorectal Cancer Lab, both at IRB Barcelona.
Of all the genes that have a relevant function, the group focused on one called Mirror in Drosophila and lrx in humans. The experiments with flies led to the finding that this gene favours tumour growth in early stages of human cancer. “The problem with human cancer is that we know very little about what happens in the early stages. Our models allows us to better study its development.” Also, Casali goes on to speculate that the human gene lrx may become a good drug target “for example, to prevent benign adenomas from developing further.” However, first the validity of the gene as a therapeutic target has to be tested in mice.
A good in vivo guinea pig for drugs
The researchers also expound that flies can be used to study candidate drug molecules to combat cancer. Drosophila would serve as an intermediate step between the in vitro phase and testing in vertebrates. On the one hand, this model has the in vitro advantages because many molecules can be tested at a minimum dose, and on the other, it shares the advantage of animals models because, as it is a living organism, toxic molecules or those with poor absorption could be omitted very quickly.
“If there are 2000 promising molecules among a million tested in vitro, instead of testing them in mice, Drosophila could offer a good alternative to identify the two or three that are most appropriate. Both time and costs would be reduced,” explains Casali.
With this aim, Casali has start collaborating with the group headed by Ernest Giralt (IRB Barcelona)—an authority on pharmacological chemistry and peptide design—to use flies to test new families of molecules against cancer.
Reference articles:
Iro/Irx transcription factors negatively regulate Dpp/TGF-b pathway activity during intestinal tumorigenesis
Òscar Martorell, Francisco M. Barriga, Anna Merlos-Suárez, Camille Stephan-Otto Attolini, Jordi Casanova, Eduard Batlle, Elena Sancho and Andreu Casali
EMBO Reports (2014 Oct 8). 10.15252/embr.201438622
Conserved mechanisms of tumorigenesis in the Drosophila adult midgut
Òscar Martorell, Anna Merlos-Suárez, Kyra Campbell, Francisco M. Barriga, Christo P. Christov,
Irene Miguel-Aliaga, Eduard Batlle, Jordi Casanova, Andreu Casali
PLoS One (2014 Feb) doi: 10.1371/journal.pone.0088413
 (1 votes)
(1 votes)
 Loading...
Loading...


 (No Ratings Yet)
(No Ratings Yet)


 (8 votes)
(8 votes)