July in preprints
Posted by the Node, on 1 August 2023
Welcome to our monthly trawl for developmental and stem cell biology (and related) preprints.
The preprints this month are hosted on bioRxiv – use these links below to get to the section you want:
- Patterning & signalling
- Morphogenesis & mechanics
- Genes & genomes
- Stem cells, regeneration & disease modelling
- Plant development
- Evo-devo
Developmental biology
| Patterning & signalling
FGF independent MEK1/2 signalling is essential for male fetal germline development in mice
Rheannon O Blucher, Rachel S Lim, Ellen G Jarred, Matthew E Ritchie, Patrick S Western
Epigenetic heterogeneity of the Notch signaling components in the human developing retina
Takahiro Nakayama, Masaharu Yoshihara, Satoru Takahashi
SH2 domain protein E (SHE) and ABL signaling regulate blood vessel size
Jennifer A. Schumacher, Zoë A. Wright, Diandra Rufin Florat, Surendra K. Anand, Manish Dasyani, Laurita Klimkaite, Nina O. Bredemeier, Suman Gurung, Gretchen M. Koller, Kalia N. Aguera, Griffin P. Chadwick, Riley D. Johnson, George E. Davis, Saulius Sumanas

Tet Controls Axon Guidance in Early Brain Development through Glutamatergic Signaling
Hiep Tran, Le Le, Badri Nath Singh, Joseph Kramer, Ruth Steward
Anastasiia Lozovska, Artemis G. Korovesi, André Dias, Alexandre Lopes, Donald A. Fowler, Gabriel G. Martins, Ana Nóvoa, Moisés Mallo
Par3/Bazooka binds NICD and promotes Notch signalling during Drosophila development
Jun Wu, Neeta Bala Tannan, Linh T. Vuong, Yildiz Koca, Giovanna M. Collu, Marek Mlodzik
Zachary M. Collins, Anna Cha, Albert Qin, Kana Ishimatsu, Tony Y.C. Tsai, Ian A. Swinburne, Pulin Li, Sean G. Megason
Shu-Yun Li, Satoko Matsuyama, Sarah Whiteside, Xiaowei Gu, Jonah Cool, Blanche Capel, Tony DeFalco
Differential susceptibility of male and female germ cells to glucocorticoid-mediated signaling
Steven A. Cincotta, Nainoa Richardson, Mariko H. Foecke, Diana J. Laird
Stefania Tavano, David B. Brückner, Saren Tasciyan, Xin Tong, Roland Kardos, Alexandra Schauer, Robert Hauschild, Carl-Philipp Heisenberg

Janani Ramachandran, Wanlu Chen, Rachel K. Lex, Kathryn E. Windsor, Hyunji Lee, Tingchang Wang, Weiqiang Zhou, Hongkai Ji, Steven A. Vokes
Neural tube organoids self-organise floorplate through BMP-mediated cluster competition
Teresa Krammer, Hannah T. Stuart, Elena Gromberg, Keisuke Ishihara, Manuela Melchionda, Jingkui Wang, Elena Costantini, Stefanie Lehr, Dillon Cislo, Laura Arbanas, Alexandra Hörmann, Ralph A. Neumüller, Nicola Elvassore, Eric Siggia, James Briscoe, Anna Kicheva, Elly M. Tanaka
| Morphogenesis & mechanics
Sung-Eun Kim, Pooja J Chothani, Rehana Shaik, Westley Pollard, Richard H Finnell
Replication Protein A1 is essential for DNA damage repair during mammalian oogenesis
Xiaosu Miao, Rui Guo, Andrea Williams, Catherine Lee, Jun Ma, P. Jeremy Wang, Wei Cui
Joaquim Grego-Bessa, Paula Gómez-Apiñaniz, Belén Prados, Manuel José Gómez, Donal MacGrogan, José Luis de la Pompa
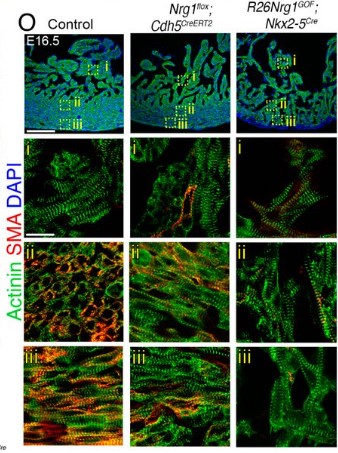
Coordination of cell cycle and morphogenesis during organ formation
Jeffrey Matthew, Vishakha Vishwakarma, Thao Phuong Le, Ryan A. Agsunod, SeYeon Chung
Zheng-Hui Zhao, Tie-Gang Meng, Fei Gao, Heide Schatten, Qing-Yuan Sun
Elise A. Loffet, John F. Durel, Richard Kam, Hyunjee Lim, Nandan L. Nerurkar
Drosophila axis extension is robust to an orthogonal pull by invaginating mesoderm
Claire M Lye, Guy B. Blanchard, Jenny Evans, Alexander Nestor-Bergmann, Bénédicte Sanson
Nadia M.E. Ayad, Johnathon N. Lakins, Ajinkya Ghagre, Allen J. Ehrlicher, Valerie M. Weaver
Leonard Drees, Dietmar Riedel, Reinhard Schuh, Matthias Behr
| Genes & genomes
The Role and Mechanism of TEAD4 in Preimplantation Embryonic Development in Mice and Cattle
Xiaotong Wu, Yan Shi, Bingjie Hu, Panpan Zhao, Shuang Li, Lieying Xiao, Shaohua Wang, Kun Zhang
Primordial germ cell DNA demethylation and development require DNA translesion synthesis
Pranay Shah, Ross Hill, Stephen Clark, Camille Dion, Abdulkadir Abakir, Mark Arends, Harry Leitch, Wolf Reik, Gerry Crossan
Karyopherin α2 is a maternal effect gene required for early embryonic development and female fertility in mice
Franziska Rother, Reinhard Depping, Elena Popova, Stefanie Huegel, Ariane Heiler, Enno Hartmann, Michael Bader
Heterogeneity and Functional Analysis of Cardiac Fibroblasts in Heart Development
Yiting Deng, Yuanhang He, Juan Xu, Haoting He, Guang Li
Temporal transcriptomic dynamics in developing macaque neocortex
Longjiang Xu, Zan Yuan, Jiafeng Zhou, Yuan Zhao, Wei Liu, Shuaiyao Lu, Zhanlong He, Boqin Qiang, Pengcheng Shu, Yang Chen, Xiaozhong Peng
| Stem cells, regeneration & disease modelling
Tim Klingberg, Irina Wachter, Agnieszka Pancholi, Yomna Gohar, Priya Kumar, Marcel Sobucki, Elisa Kämmer, Süheyla Eroğlu-Kayıkçı, Sylvia Erhardt, Carmelo Ferrai, Vasily Zaburdaev, Lennart Hilbert
Sara Ahmed-de-Prado, Carlos Estella, Antonio Baonza

James L. Engel, Xianglong Zhang, Daniel R. Lu, Olaia Fernandez Vila, Vanessa Arias, Jasper Lee, Christopher Hale, Yi-Hsiang Hsu, Chi-Ming Li, Vasanth Vedantham, Yen-Sin Ang
Nathaniel K. Mullin, Laura R. Bohrer, Andrew P. Voigt, Lola P. Lozano, Allison Wright, Robert F. Mullins, Edwin M. Stone, Budd A. Tucker
Human receptive endometrial organoid for deciphering the implantation window
Yu Zhang, Rusong Zhao, Chaoyan Yang, Jinzhu Song, Peishu Liu, Yan Li, Boyang Liu, Tao Li, Changjian Yin, Minghui Lu, Zhenzhen Hou, Chuanxin Zhang, Zi-Jiang Chen, Keliang Wu, Han Zhao
Connexin 41.8 mediates the correct temporal induction of haematopoietic stem and progenitor cells
Tim Petzold, Masakatsu Watanabe, Julien Y. Bertrand
Macrophages heterogeneity and significance during human fetal pancreatic development
Adriana Migliorini, Sabrina Ge, Michael H. Atkins, Rangarajan Sambathkumar, Angel Sing, Conan Chua, Adam J. Gehring, Gordon M. Keller, Faiyaz Notta, Maria Cristina Nostro
Development of a hepatic cryoinjury model to study liver regeneration
Marcos Sande-Melon, David Bergemann, Juan Manuel González-Rosa, Andrew Cox
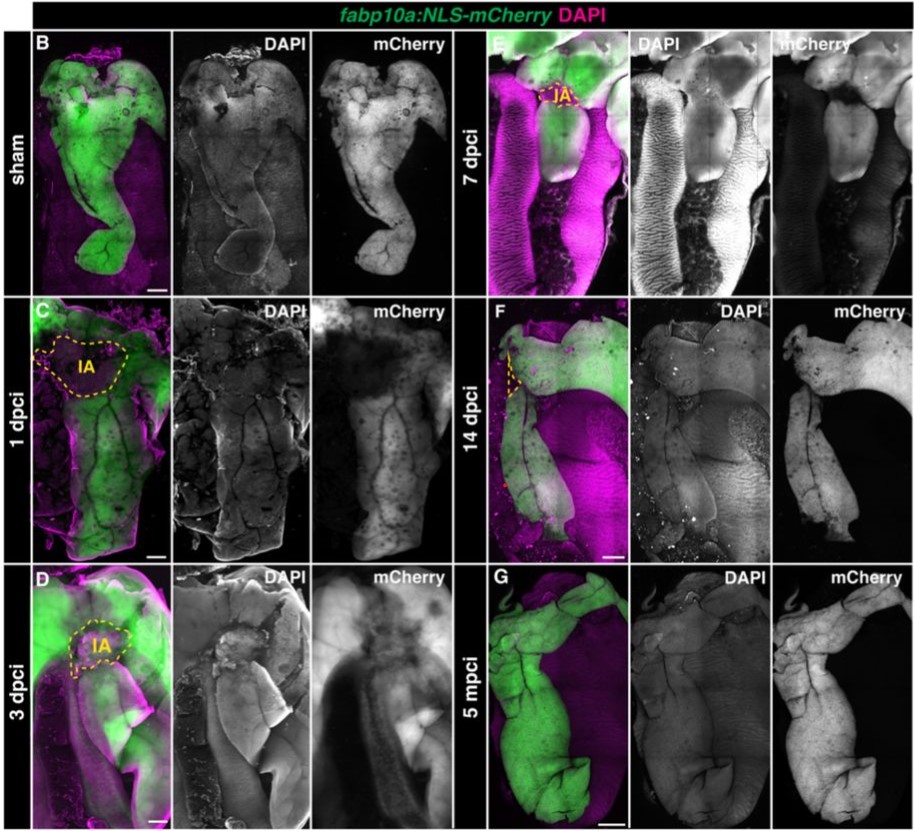
Modeling Human Spine-Spinal Cord Organogenesis by hPSC-Derived Neuromesodermal Progenitors
Dairui Li, Yuanchen Ma, Weijun Huang, Xiaoping Li, Huanyao Liu, Chuanfeng Xiong, Qi Zhao, Bin Wang, Xingqiang Lai, Shanshan Huang, Yili Wei, Junhua Chen, Xiyu Zhang, Lan Wei, Wenjin Ye, Qiumin Chen, Limin Rong, Andy Peng Xiang, Weiqiang Li
Zaid Muhammad, Phoebe W. Brown, Larema Babazau, Abdulrahman I. Alkhamis, Baba W. Goni, Haruna A. Nggada, Kefas M. Mbaya, Selina Wray, Isa H. Marte, Celeste M. Karch, Louise C. Serpell, Mahmoud B. Maina
Ádám Póti, Dávid Szüts, Jelena Vermezovic
ERK signalling orchestrates metachronous transition from naïve to formative pluripotency
Carla Mulas, Melanie Stammers, Siiri I. Salomaa, Constanze Heinzen, David M. Suter, Austin Smith, Kevin J. Chalut
Chee Ho H’ng, Shanika L. Amarasinghe, Boya Zhang, Hojin Chang, David R. Powell, Alberto Rosello-Diez
Birthe Dorgau, Joseph Collin, Agata Rozanska, Veronika Boczonadi, Marina Moya-Molina, Rafiqul Hussain, Jonathan Coxhead, Tamil Dhanaseelan, Lyle Armstrong, Rachel Queen, Majlinda Lako
| Plant development
Asaka Kanda, Kento Otani, Takashi Ueda, Taku Takahashi, Hiroyasu Motose

Benoit Daviet, Christian Fournier, Llorenc Cabrera-Bosquet, Thierry Simonneau, Maxence Cafier, Charles Romieu
Mika Tei, Fumiyuki Soma, Ettore Barbieri, Yusaku Uga, Yosuke Kawahito
An Enhancer Trap system to track developmental dynamics in Marchantia polymorpha
Alan O. Marron, Susana Sauret-Gueto, Marius Rebmann, Linda Silvestri, Marta Tomaselli, Jim Haseloff
Phanu T. Serivichyaswat, Abdul Kareem, Ming Feng, Charles W. Melnyk
Chitosan stimulates root hair callose deposition and inhibits root hair growth
Matēj Drs, Samuel Haluška, Eliška Škrabálková, Pavel Krupař, Lucie Brejšková, Karel Muller, Natalia Serrano, Andrea Potocká, Aline Voxeur, Samantha Vernhettes, Jitka Ortmannová, George Caldarescu, Matyas Fendrych, Martin Potocký, Viktor Žárský, Tamara Pečenková
Edouard Tourdot, Elie Maza, Anis Djari, Pascal GP Martin, Frederic Gevaudant, Christian Chevalier, Julien Pirrello, Nathalie Gonzalez
Harnessing Deep Learning to Analyze Cryptic Morphological Variability of Marchantia polymorpha
Yoko Tomizawa, Naoki Minamino, Eita Shimokawa, Shogo Kawamura, Aino Komatsu, Takuma Hiwatashi, Ryuichi Nishihama, Takashi Ueda, Takayuki Kohchi, Yohei Kondo
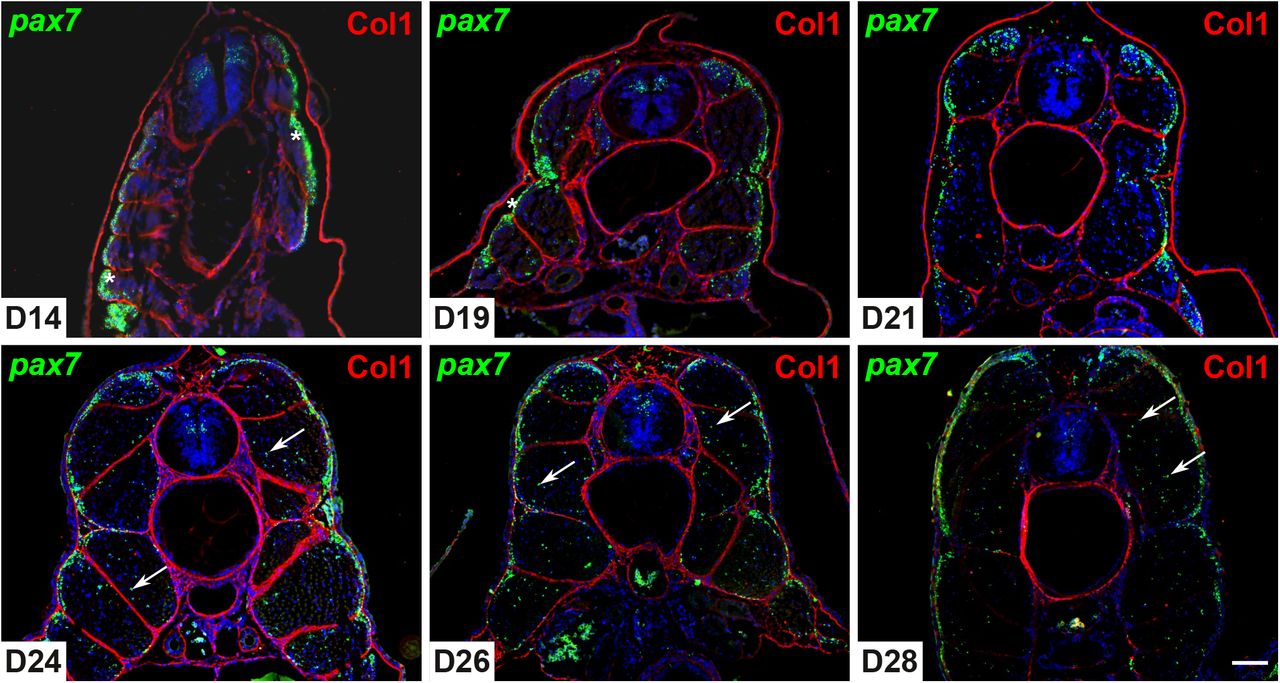
Growth directions and stiffness across cell layers determine whether tissues stay smooth or buckle
Avilash S. Yadav, Lilan Hong, Patrick M. Klees, Annamaria Kiss, Xi He, Iselle M. Barrios, Michelle Heeney, Anabella Maria D. Galang, Richard S. Smith, Arezki Boudaoud, Adrienne H.K. Roeder
Pauline Delpeuch, Sophie Nadot, Katia Belcram, Antoine Plumerault, Catherine Damerval, Florian Jabbour
Development of a mobile, high-throughput, and low-cost image-based plant growth phenotyping system
Li’ang Yu, Hayley Sussman, Olga Khmelnitsky, Maryam Rahmati Ishka, Aparna Srinivasan, Andrew D.L. Nelson, Magdalena M. Julkowska
Expression of cell-wall related genes is highly variable and correlates with sepal morphology
Diego A. Hartasánchez, Annamaria Kiss, Virginie Battu, Charline Soraru, Abigail Delgado-Vaquera, Florian Massinon, Marina Brasó-Vives, Corentin Mollier, Marie-Laure Martin-Magniette, Arezki Boudaoud, Françoise Monéger
| Evo-devo
An ancient split of germline and somatic stem cell lineages in Hydra
Chiemi Nishimiya-Fujisawa, Hendrik Petersen, Tracy Chih-Ting Koubková-Yu, Chiyo Noda, Shuji Shigenobu, Josephine Bageritz, Toshitaka Fujisawa, Oleg Simakov, Satoru Kobayashi, Thomas W. Holstein
DNA conserved in diverse animals since the Precambrian controls genes for embryonic development
Martin C. Frith, Shengliang Ni
Developmental noise and phenotypic plasticity are correlated in Drosophila simulans
Keita Saito, Masahito Tsuboi, Yuma Takahashi
Divergent evolution of head morphology between marine and freshwater sticklebacks
Antoine Fraimout, Ying Chen, Kerry Reid, Juha Merilä
Multiple embryonic sources converge to form the pectoral girdle skeleton in zebrafish
Shunya Kuroda, Robert L. Lalonde, Thomas A. Mansour, Christian Mosimann, Tetsuya Nakamura
Janet Wei, Thomas W.P. Wood, Kathleen Flaherty, Alyssa Enny, Ali Andrescavage, Danielle Brazer, Dina Navon, Thomas A. Stewart, Hannah Cohen, Anusha Shanabag, Shunya Kuroda, Ingo Braasch, Tetsuya Nakamura
Cell Biology
Aaron Z.A. Schwartz, Yusuff Abdu, Jeremy Nance
Burak Demirbas, Olga Filina, Timo Louisse, Yvonne Goos, María Antonia Sánchez-Romero, María Olmedo, Jeroen van Zon
P Tignard, K Pottin, A Geeverding, M Doulazmi, M Cabrera, C Fouquet, M Liffran, A Trembleau, MA Breau
Allison E Hall, Diana Klompstra, Jeremy Nance
Global decoupling of cell differentiation from cell division in early embryo development
Kalki Kukreja, Nikit Patel, Sean G Megason, Allon M Klein
Actin-Driven Nanotopography Enhances Integrin Molecular Clutch in Developing Tissue
Tianchi Chen, Cecilia Huertas Fernández-Espartero, Abigail Illand, Ching-Ting Tsai, Yang Yang, Benjamin Klapholz, Pierre Jouchet, Mélanie Fabre, Olivier Rossier, Bianxiao Cui, Sandrine Lévêque-Fort, Nicholas H. Brown, Grégory Giannone
Khanh M. Vien, Qichen Duan, Chun Yeung, Pelin C Volkan
Victoria E. Deneke, Andreas Blaha, Yonggang Lu, Jonne M. Draper, Clara S. Phan, Karin Panser, Alexander Schleiffer, Laurine Jacob, Theresa Humer, Karel Stejskal, Gabriela Krssakova, Dominik Handler, Maki Kamoshita, Tyler D.R. Vance, Elisabeth Roitinger, Jeffrey E. Lee, Masahito Ikawa, Andrea Pauli
Ai Kiyomitsu, Toshiya Nishimura, Shiang Jyi Hwang, Satoshi Ansai, Masato T. Kanemaki, Minoru Tanaka, Tomomi Kiyomitsu
Cécile Rallière, Sabrina Jagot, Nathalie Sabin, Jean-Charles Gabillard
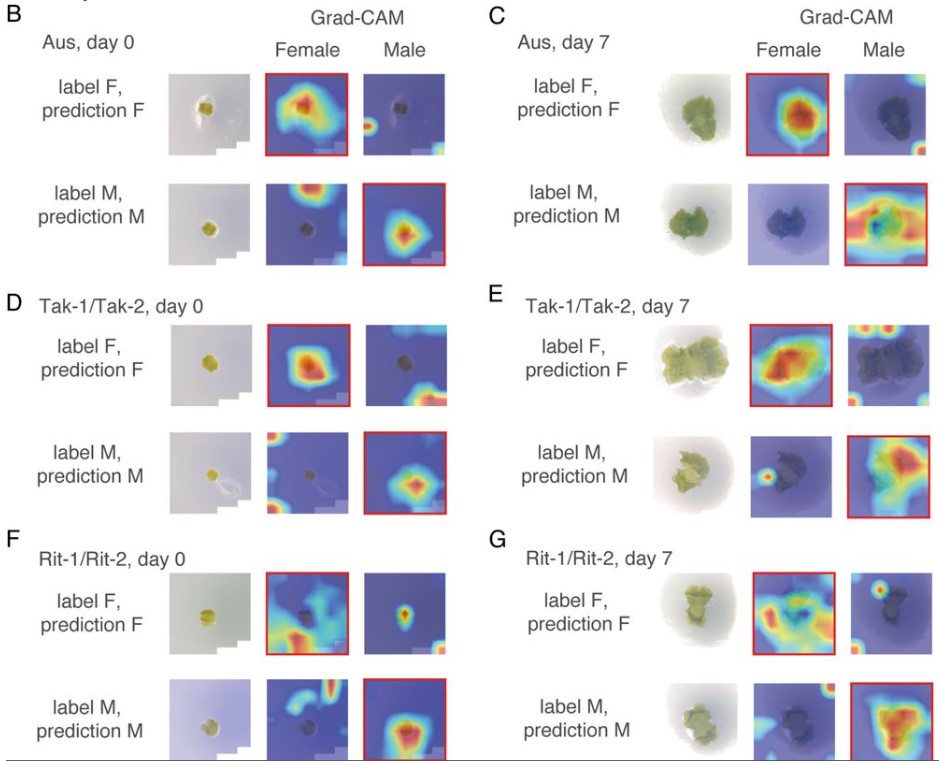
Adam Ahmad, Yoel Bogoch, Gal Shvaizer, Yaniv M. Elkouby
Jiyeon Jeong, Inchul Choi
Trophoblast organoids with physiological polarity model placental structure and function
Liheng Yang, Pengfei Liang, Huanghe Yang, Carolyn B. Coyne
Role of trafficking protein particle complex 2 in medaka development
Francesca Zappa, Daniela Intartaglia, Andrea M. Guarino, Rossella De Cegli, Cathal Wilson, Francesco G. Salierno, Elena Polishchuk, Nicolina Cristina Sorrentino, Ivan Conte, Maria Antonietta De Matteis
Rho-associated kinase regulates Langerhans cell morphology and responsiveness to tissue damage
Eric Peterman, Elgene J.A. Quitevis, Camille E.A. Goo, Jeffrey P. Rasmussen
Modelling
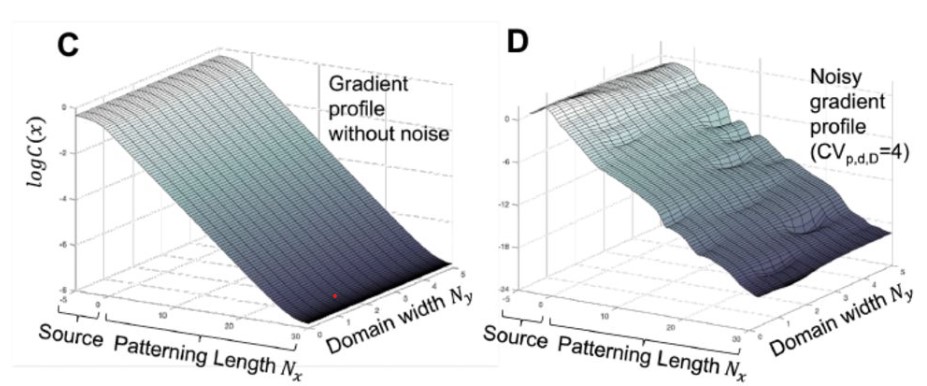
2D Effects Enhance Precision of Gradient-Based Tissue Patterning
Yuchong Long, Roman Vetter, Dagmar Iber
Alexandra Nicole Taylor, Rachel Lockridge Mueller, Ashok Prasad
Tools & Resources
MICA: A multi-omics method to predict gene regulatory networks in early human embryos
Gregorio Alanis-Lobato, Thomas E Bartlett, Qiulin Huang, Claire Simon, Afshan McCarthy, Kay Elder, Phil Snell, Leila Christie, Kathy Niakan
Matthieu Duot, Roselyne Viel, Justine Viet, Catherine Le Goff-Gaillard, Luc Paillard, Salil Lachke, Carole Gautier-Courteille, David Reboutier
Jared A Tangeman, Sofia M Rebull, Erika Grajales-Esquivel, Jacob M Weaver, Stacy Bendezu-Sayas, Michael L Robinson, Salil A Lachke, Katia Del Rio-Tsonis
Daniel J Leite, Anna Schoenauer, Grace Blakeley, Amber Harper, Helena Garcia-Castro, Luis Baudouin-Gonzalez, Ruixun Wang, Naira Sarkis, Alexander Gunther Nikola, Ventaka Sai Poojitha Koka, Nathan J Kenny, Natascha Turetzek, Matthias Pechmann, Jordi Solana, Alistair P McGregor
OrgaMapper: A robust and easy-to-use workflow for analyzing organelle positioning
Christopher Schmied, Michael Ebner, Paula Samsó Ferré, Volker Haucke, Martin Lehmann
A matrisome atlas of germ cell development
Aqilah Amran, Lara Pigatto, Johanna Farley, Rasoul Godini, Roger Pocock, Sandeep Gopal
Angelica Miglioli, Marion Tredez, Manon Boosten, Camille Sant, João E. Carvalho, Philippe Dru, Laura Canesi, Michael Schubert, Rémi Dumollard
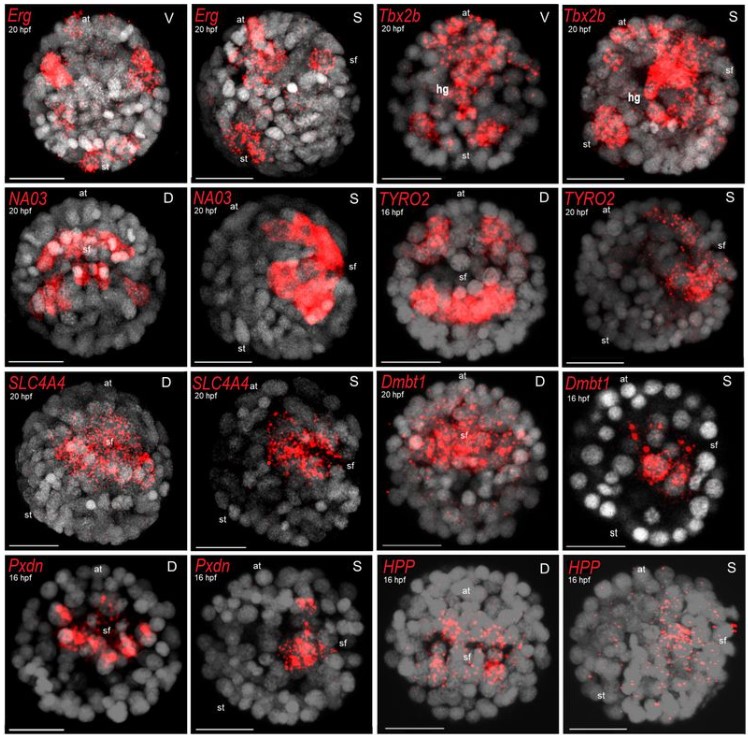
Modelling Human Post-Implantation Development via Extra-Embryonic Niche Engineering
Joshua Hislop, Amir Alavi, Qi Song, Rayna Schoenberger, Kamyar Keshavarz F., Ryan LeGraw, Jeremy Velazquez, Tahere Mokhtari, Mohammad Nasser Taheri, Matthew Rytel, Susana M Chuva de Sousa Lopes, Simon Watkins, Donna Stolz, Samira Kiani, Berna Sozen, Ziv Bar-Joseph, Mo R. Ebrahimkhani
Methods for cell isolation and analysis of the highly regenerative tunicate Polycarpa mytiligera
Tal gordon, Noam Hendin, Omri Wurtzel
Spinal neural tube formation and regression in human embryos
Chloe Santos, Ailish Murray, Abigail R. Marshall, Kate Metcalfe, Priyanka Narayan, Sandra C. P. de Castro, Eirini Maniou, Nicholas D. E. Greene, Gabriel L. Galea, Andrew J. Copp
Research practice & education
Purchases dominate the carbon footprint of research laboratories
Marianne De Paepe, Laurent Jeanneau, Jerôme Mariette, Olivier Aumont, Andŕe Estevez-Torres
ChatGPT identifies gender disparities in scientific peer review
Jeroen P. H. Verharen
Angelica Monarrez, Lourdes Echegoyen, Danielle Morales, Diego Seira, Maria Aleida Ramirez, Amy Wagler
Origami-based growing tube model for reproducing shell shapes
Maho Ueda, Nozomi Fukunaga, Noa Yamashita, Yuki Yokoyama, Hideaki Kida, Keisuke Matsuda
Chrisostomos Drogaris, Alexander Butyaev, Elena Nazarova, Roman Sarrazin-Gendron, Harsh Patel, Akash Singh, Brenden Kadota, Jérôme Waldispühl
Patrick D. Brandt, Dawayne Whittington, Kimberley D. Wood, Chris Holmquist, Ana T. Nogueira, Christiann H. Gaines, Patrick J. Brennwald, Rebekah L. Layton
Internet-connected cortical organoids for project-based stem cell and neuroscience education
Matthew A.T. Elliott, Hunter E. Schweiger, Ash Robbins, Samira Vera-Choqqueccota, Drew Ehrlich, Sebastian Hernandez, Kateryna Voitiuk, Jinghui Geng, Jess L. Sevetson, Yohei M. Rosen, Mircea Teodorescu, Nico O. Wagner, David Haussler, Mohammed A. Mostajo-Radji
Unreviewed science in the news: The evolution of preprint media coverage from 2014-2021
Alice Fleerackers, Kenneth Shores, Natascha Chtena, Juan Pablo Alperin
Bhavesh Patel, Sanjay Soundarajan, Hervé Ménager, Zicheng Hu


 (4 votes)
(4 votes)

 (No Ratings Yet)
(No Ratings Yet)