One more day to vote!
Posted by the Node, on 20 June 2017
Our image competition for a future Development cover is still live: voting closes tomorrow at 13.00 GMT! Click here to vote and find out more about the images
Posted by the Node, on 20 June 2017
Our image competition for a future Development cover is still live: voting closes tomorrow at 13.00 GMT! Click here to vote and find out more about the images
Posted by Gregg Duester, on 20 June 2017
Closing Date: 15 March 2021
An NIH-funded postdoctoral position is available to investigate the signaling functions of retinoic acid (RA) during mouse embryo development. Our laboratory has reviewed recent advances in this field: Cunningham, T.J. and Duester, G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nature Reviews Molecular Cell Biology 16: 110-123 (2015).
We are searching for a Postdoctoral Associate to explore the mechanisms of RA signaling and nuclear receptor coregulators during the early stages of organogenesis and limb formation using CRISPR/Cas9 gene editing, RNA-seq transcriptome analysis, and embryo chromatin immunoprecipitation.
Candidates should have a strong background in developmental biology or stem cell biology. Salary $47,476 with full benefits.
Interested applicants should email their CV and names of three references to:
Gregg Duester, Ph.D.
Professor
Development, Aging, and Regeneration Program
Sanford Burnham Prebys Medical Discovery Institute
10901 North Torrey Pines Road
La Jolla, California 92037, USA (San Diego area)
For more information please visit the Lab Website:
Posted by radikes, on 19 June 2017
Things are in full swing at MBL in the 2017 Embryology course, we are already one third of the way through! The first week we learned about echinoderms and C. elegans with a side of Tardigrade and this past we focused on zebrafish and Xenopus with axolotl. The atmosphere of the past two weeks has been full of excitement as we learn about new model systems and techniques. Not only did we discover new things scientifically, but we also learned more about each other as students and faculty.
A key element that appeared this week is our connection to each other and to the generation of scientists before us. It has been breath taking to learn about all the contributions that individuals and groups made to the field and to hear that so many of these discoveries were made either here at Woods Hole or by scientists who were a part of the course at some point during their career. It is thrilling to think that we are establishing meaningful connections, collaborations, and friendships that will be with us for the rest of our lives!
What enhances the connection is the unique diversity in the group, we all come from different places and have different backgrounds. The group is a mix of developmental, cell, and computational biologists as well as bioinformaticians, biophysicists and engineers. These differences in training and background paired with our general passion for science and inquiry have allowed us to look at problems from new and different angles. This is exciting, enriching and stimulates us as a group to propose and test new hypothesis.
For up to date info on the course follow us on Twitter #embryo2017 or instagram #embryology2017
Posted by Reena Lasrado, on 16 June 2017
Insight into the organizational structure of a growing tissue is imperative for understanding its development and function. Structure can reveal the systematic steps undertaken towards making specific positional and cell fate choices/decisions. A well-defined structure helps to dissect the complexity underlying the networks that form as the tissue develops. It helps to elucidate the foundation on which the components can work together within the confines of the system. It helps one to read the lines of communication that are built into the system for its proper functioning. When an organized structure regulates the flow of information, then changes within that information are easier to monitor and adapt.
Historical views
The Enteric Nervous System (ENS) is one of the largest subdivisions of the peripheral nervous system. It earns its’ name as the “second brain” due to the millions of neurons it contains and the complex neural networks it displays1. Although Bayliss and Starling’s description of ‘the law of the intestine’ hallmarked the functional discovery of the ENS already more than a century ago,2 their discovery also unveiled some daunting scientific challenges, many of which are explained by inadequate insight into ENS structure and how this contributes to its function.
Simply put – It is a very challenging system for various different reasons. The ENS is challenged every day with the variety of food/drink we intake, which means the environment is under constant change. The ENS has a mind of its own! It autonomously controls functions of the gut that include peristalsis, secretion of enzymes and absorption of food. The ENS is layered in close apposition to contractile sheets of smooth muscle cell syncytia, thereby complicating several experimental approaches to a large extent. The gut lumen is host to a vast ocean of microbes with which the ENS interacts3 with other systems within this tissue, such as, the immune and epithelial systems to maintain a healthy gut. In addition, it also communicates with the brain to keep the body in good health.
The ENS originates from neural crest-derived progenitors that travel through different spatio-temporal environments as they expand in number, colonize the gut tissue and differentiate to generate a plethora of neuronal and glial cells. Unlike the central nervous system, neurons and glial cells of the ENS do not show any apparent order of arrangement. Cells coalesce in a salt and pepper manner to form ganglia and create an expansive network. However, reproducible patterns of secretory and motor function appear to be almost exclusively controlled by the ENS. Being indispensable for gut physiology, its lack of function is implicated in the pathogenesis of several gastrointestinal disorders, such as Hirschsprung disease and others that are of unknown aetiology.
Important questions
For a system that is tremendously interactive in an extensively expanding tissue (8 metres in humans), knowledge of how its structure develops is very important. This information can be useful in understanding diseases of the gut that may arise during development or later in adulthood and disrupt the network. Therefore, it was imperative for us to make sense of the ‘randomness’ that the ENS displays. We wished to gain an understanding of any fundamental rules that defined its apparent chaotic cellular topology and eventually its contribution to function.
Previous work in many labs including ours, have aimed to understand ENS structure and function by using global approaches and studying it at the population level. However, such approaches have not been very successful in gaining detailed insight into how the lineages of the ENS develop and contribute to its structure and function. As a lab that carries a reputation of developing tools and techniques that help to answer big over-arching questions in the field, we took the plunge. We decided to opt for single-cell approaches so as to address the contribution of an individual progenitor to the spatial development and function of the ENS at both the cellular and molecular level.
The work
Appropriate combination of genetic tools4 were generated in the lab and employed to track the static behaviour of individual ENS progenitors in a multi-colour mosaic manner over the developmental time line. Having optimized the various steps involved in the different experiments our results looked promising enough for me to spend hours and days to localize and image the beautiful clones we obtained in our study. The scanned clones were analysed for their composition, measured left, right and centre to gain an understanding about their spatio-temporal characteristics. We combined in vivo and ex-vivo approaches to capture the cellular properties of these cells. The days spent away from imaging were invested in isolating single cells for the transcriptomic study. This analysis was performed to understand the intrinsic properties of heterogeneous individual progenitors and how ENS lineages are generated. We also used mosaic mutagenesis to disrupt the system during development and understand its effect on ENS composition.
Making sense
With this arduous approach the mysterious topology of the ENS unfolded before our eyes. The close spatial relationship of the labelled families both at the 2D and 3D axes with a single colour meant ‘something’ to the system. This study helped us to unravel a set of rules that define the columnar organization of overlapping clonal lineages. This suggested to us that this structural organization could help the components of the ENS to work and face the challenges of the system together as a family. We also observed that as the system developed, subpopulations emerged in a defined manner. Neurons related to each other showed co-ordinate activity upon stimulation, highlighting their means of communication within the ENS of the small intestine. Molecular analysis of ENS progenitors revealed the manner in which neuronal and glial lineages arise. Further, our mutagenesis study revealed the specific role of an important receptor tyrosine kinase, RET in neuronal commitment. Together, our work suggests that lineage relationships are fundamental for the spatial organization and function of the ENS.
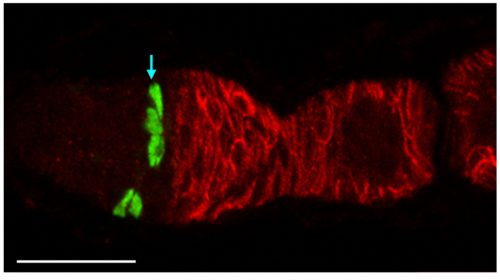
Neuro-glial clone: This image shows the progeny of a neuro-glial progenitor in the myenteric plexus of the adult mouse. Neurons extend processes forming a meshwork of connectivity and glial cells are observed within ganglia.
Future
Perhaps mistakes in the blueprint of the ENS are the cause of gastrointestinal diseases with unknown aetiology. We are yet to uncover the principles that underlie information processing, which will help us to assign the logic of ENS assembly and connectivity. Now that we have a better understanding of how the ENS of the small intestine assembles and underpins its function, we can start to probe this system at different stages of development.
This blog is contributed by Reena Lasrado and Werend Boesmans.
References
Posted by Noami Dayan, on 16 June 2017
Closing Date: 15 March 2021
We are seeking a highly motivated and ambitious candidate to join the research activities within our stem cell and developmental biology projects.
Posted by Amy Ruth Reilein, on 16 June 2017
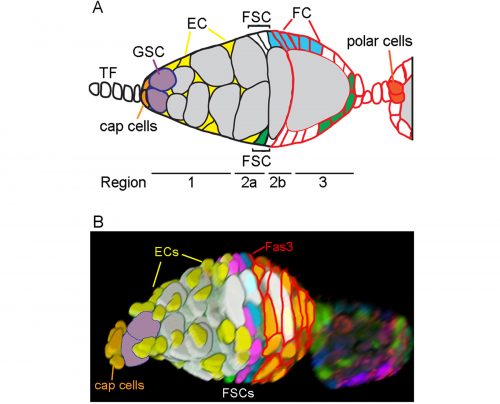
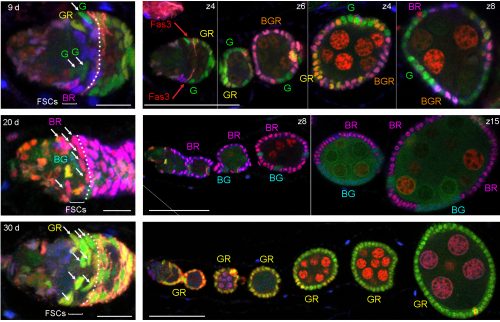
We have recently revised the model of Follicle Stem Cell (FSC) organization in the Drosophila ovary, showing that there is a much larger population of stem cells than formerly realized, that these FSCs exhibit population asymmetry, and that they give rise to Escort Cells as well as Follicle Cells [1]. Ovarian Germline Stem Cells have long been used as model stem cells, benefiting from easy recognition by virtue of their characteristic location at the anterior of the germarium, morphology, and functional markers that reflect the actions of a key BMP (bone morphogenetic protein) niche signal. FSCs, which are required for the development of germline cells into a mature oocyte, have been more difficult to investigate. No marker specific to these stem cells has been discovered, they are not in an easily recognized location and are not morphologically conspicuous among their neighbors.
In 1995 Margolis and Spradling reported the identification of FSCs (originally named Somatic Stem Cells), which they located midway through the germarium by BrdU labeling and lineage analysis [2]. An FSC is defined by lineage analysis as a cell that can produce Follicle Cells (FCs) but remains in the germarium while germline cysts pass through. FSC derivatives that associate with a germline cyst in the posterior half of the germarium all initially proliferate. A few quickly become specialized post-mitotic polar cells and stalk cells to allow egg chamber budding from the germarium, while the rest form an expanding epithelium around the germline cyst, cease divisions in mid-size egg chambers (stage 6) and adopt position-specific fates about a day later (stage 9). We refer here to all of these FSC derivatives including the earliest precursors (often termed prefollicle cells) associated with a germline cyst as Folicle Cells (FCs). Marked lineages that originate in an FC have a predictable, limited lifespan because all FC progeny move through the germarium and ovariole along with the associated cyst at an established rate. It takes the earliest FC about two days to exit the germarium and five days to exit the ovariole. If a marked cell remains in the germarium or the ovariole for longer than this time then we can deduce that the lineage must have originated in an FC precursor, namely an FSC. Although this definition has been used since 1995, our perception has changed from expecting most FSCs to reside in the germarium much longer than an FC, to realizing that many FSCs are in fact very short-lived before becoming FCs.
History of FSC numbers
The number of stem cells was first calculated as the reciprocal of the fraction of the FC epithelium covered by a marked FSC clone and was measured at 9-11 days after clone induction to be sure that all transient FC clones had exited the ovariole [2]. Care was taken that the FSC clones examined likely originated from a single marked cell by inducing clones at low frequency with a heat-shock inducible recombination event, but an implicit assumption was made that FSCs divide asymmetrically (to produce one stem cell and one non-stem cell at each division) and that there would therefore be one marked FSC throughout the history of generating marked FCs. At the time, this was a common assumption for stem cells, inspired in part by studies of ovarian Germline Stem Cells. We found that FSCs do not repeatedly undergo asymmetric divisions but rather exhibit frequent amplification or loss of individual lineages (see later). An accurate count of FSCs deduced from FC contributions cannot therefore be made at 9 days after clone induction because by this time a single surviving clone will include an unknown number of FSCs. Earlier results therefore reflect the FC contribution of multiple FSCs, leading to a large underestimate of the total number of FSCs.
In 2001, Zhang and Kalderon used 3 genetic lineage labels and detected up to 3 different FSC lineage colors in a single ovariole 8 days after clone induction, concluding that there were likely three FSCs [3]. Identification of an FSC lineage was constrained by the requirement to identify an FC-contributing, surviving FSC in a location roughly consistent with the two FSC positions mapped by Margolis and Spradling; any marked FSCs that were lost prior to 8d, that occupied an unexpected position or did not contribute any FCs over the last five days would not have been scored. In 2007 Nystul and Spradling also used 3-genotype labeling and postulated 2 fixed niches of FSCs on opposite sides of the germarium, stating that the germarium is bilaterally symmetric and lies down on the slide in a particular orientation such that the two FSC niches are on either side of the germarium [4]. They did sometimes see three FC genotypes in an ovariole, but postulated that an FSC daughter could temporarily occupy one of the niches and act as an FSC.
In our more recent experiments we used the same strategies as before (counting the contribution of a single FSC lineage to the FC epithelium or counting the number of distinctly colored FSC lineages that can be generated in a single epithelium) but concluded that there are many more FSCs. The biggest reason for the very different outcome was the consideration that some FSC lineages might be lost very quickly while others amplify, necessitating looking at outcomes at a variety of times after marking. Other important differences included using more colors for lineage marking; consideration of more potential locations for FSCs and the possibility that a marked FSC may only occasionally give rise to an FC; and thorough analysis of all cells in a set of ovarioles selected without bias. We developed a lineage tracing method that yields 6 different combinations of GFP, RFP and β-galactosidase. Our strategy was to induce as many recombinations as possible in dividing stem cells in order to see the maximum number of FSC lineages. Using the enduring definition of a surviving marked FSC (a cell that remains in the 2a/b region and has given rise to at least one FC patch in the same ovariole), we found up to six (the maximum we could score) surviving FSC lineages in a single ovariole and inferred from the number of colors seen in a large sample of ovarioles that there were about five surviving FSC lineages on average.
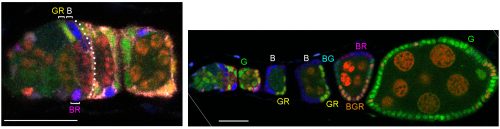
We also found that a single FSC lineage contributed to about 15% of the FC epithelium in a highly mosaic fashion with zero contribution to several egg chambers. Most important, counting surviving FSC lineages at different times after marking showed that many more lineages were present at 5d, while there were decreasing numbers at later times. We estimated that there may be about ten surviving FSC lineages at 5d, but we would need more than six color combinations to count more accurately (five or six colors were present in almost all ovarioles). Consequently, our extrapolation that there may have originally been as many as sixteen FSC lineages at day zero is also just an approximation. In these experiments we did not change the basic methodology or logic of deducing FSC numbers: every one of the cells we called an FSC remained in the germarium for longer than an FC can and had produced at least one FC, defining it unequivocally as an FSC.
Even after taking stem cell loss and amplification into account, it turns out that there is a further limitation on what was counted as a functional FSC because we required proof that an FSC produced an FC. It turns out that some of the roughly sixteen FSCs in a germarium, all of which we believe to have the potential to produce FCs, do not produce any FCs in the 4-5 day time span displayed on a fixed ovariole. This stems in essence from the apparently stochastic behavior of FSCs and positional heterogeneity within the FSC population.
FSCs are radially distributed in layers
In order to identify positions of FSCs we had to examine only those FSC clones with a single candidate stem cell (a single FSC of a certain color that matched an FC patch). This approach allowed us to define multiple locations for an FSC anywhere around the circumference of the germarium and in any of three anterior-posterior (A/P) layers adjacent to the Fasciclin 3-expressing FCs.
One of the challenges we faced as we started to realize that there were many more FSCs than expected was how to score them in sufficient detail. Eventually we developed spreadsheets to record, for each germarium and ovariole, every cell in the 2a/b region by A/P position, and the proportion of each egg chamber occupied by each color. This level of detail was essential to see where FSCs are located and to measure contributions to the FC epithelium. Tabulating the total number of cells in the 2a/b region where FSCs could be found, “the FSC region,” (16 cells on average) provided a second means to deduce the total number of FSCs. The frequency of clones with a single FSC in a given layer was very similar to the distribution of all somatic cells in the FSC region (about 8/16 in layer 1, 6/16 in layer 2 and 2/16 in layer 3, on average), suggesting that all of the roughly 16 cells are FSCs.
At the same time that we started scoring FSCs around the entire circumference, we were surprised to find by live imaging that cells in the FSC region do not stay in fixed locations but undergo back-and-forth radial movements. In addition, the ring of cells around the circumference is indistinguishable when examined by many Gal4 expression patterns and markers [5]. The existence of apparently identical interchangeable cells around the circumference of the germarium at a given A/P position suggests that each ring of FSCs constitutes a pool of equivalent cells.
Population asymmetry
Fundamental to revising our ideas about FSC numbers and behavior is the realization that they are governed by population asymmetry. Population asymmetry is generally understood to mean that the daughters of a stem cell do not necessarily have different fates (one stem and one non-stem cell). Instead, within a homogeneous stem cell population of fixed size, individual stem cells undergo neutral competition during which a stem cell is frequently and stochastically duplicated or lost. Short-lived stem cells are not pre-determined or different from long-lived stem cells—they are selected by chance. If you look at the starting population you can say that only a small subset of those cells will survive as lineages for long time periods but you don’t know which ones. Hence, all of the cells are equivalent and have the potential to survive for a long time and are appropriately given the same name—stem cells. One would have to look very early after marking stem cells to capture them all before any are lost, which is often not practical. Counting the lineages after an arbitrary delay yields the number of stem cell lineages that survived for that time, and eventually only a single lineage will remain. Population asymmetry is by now a well-recognized arrangement that applies to many stem cells, including mammalian gut stem cells[6, 7]. In the mouse small intestine there are around 16 stem cells per crypt but several weeks after labeling individual cells with multiple colors only 1-3 lineages remain in most crypts[7]. We have exactly analogous findings. In both cases researchers first looked long after stem cell marking and, accordingly, thought there were a smaller number of stem cells. The appreciation of the dynamics of population asymmetry resolves these apparent contradictions in both cases.
Our data counting colored patches in ovarioles with no corresponding FSC at 9d clearly showed that many FSC lineages are lost rapidly. By 30 days the majority of ovarioles are monoclonal (we found an average of 1.5 lineages out of 60 ovarioles scored). There is a corresponding increase in the number of FSCs per lineage over time. This demonstration of rapid FSC lineage loss along with a corresponding increase in the number of 2a/b cells per surviving lineage over time shows that FSCs are maintained by population asymmetry.
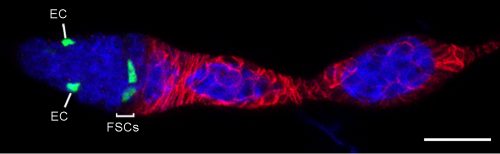
Origin of Escort Cells—why had it been difficult to see the relationship to FSCs?
ECs are also labeled in lineage experiments in adults, showing that they are renewed in adult ovaries. However, Margolis and Spradling concluded that there is no lineage relationship between FSCs and ECs [2]. Kirilly et al. concurred but found that the source of new ECs is region 2a/b, very close to FSCs, postulating that the dividing cells are themselves ECs [8]. In these and other lineage studies, including ours, one could readily see ovarioles with marked FCs but no ECs, or marked ECs but no FCs, but there were also many ovarioles containing marked ECs and FCs (as well as FSCs). It was challenging to unravel the basis of these different outcomes.To prove that an FSC can give rise to both ECs and FCs it is essential to prove that a marked lineage containing all three cell types derived from a single cell. That is normally accomplished by inducing marked cells at very low frequency. We found it difficult to label single FSCs using the MARCM technique even by using the mildest of heat-shocks. It is understandable that it is quite difficult to target a single FSC for recombination by chance, given that there are about 16, rather than 2-3 per germarium. We eventually accomplished a satisfactorily low clone induction by additionally taking advantage of multicolor labeling to produce a very low frequency of clones with a specific color combination.
We also became convinced that all adult-born ECs derived from FSCs because the locations of cells in region 2a/b that proliferate, according to EdU labeling, is the same as FSC locations deduced from our multicolor labeling; it was previously thought that most of these cells were ECs. We can now categorize an EC not only by location and morphology but also by the unifying property of exhibiting no proliferation. However, it was puzzling that many lineages contained only ECs or only FCs if all of their progenitors can give rise to both ECs and FCs. We resolved this paradox by a combination of careful analysis of FSC lineages over time and the appreciation that the FSC population is heterogeneous. FSCs can produce only FCs for a few days in succession but a longer time-course shows that virtually all FC-producing lineages eventually produce ECs. Conversely, FSCs can produce only ECs for a few days; ovarioles only reveal FC production over the past 4-5 days, so a constant fraction of FSCs (around 20%) are not associated with marked FCs even though FC production may have been in their past or future. The explanation for periods of producing only ECs or only FCs lies in positional heterogeneity of FSCs.
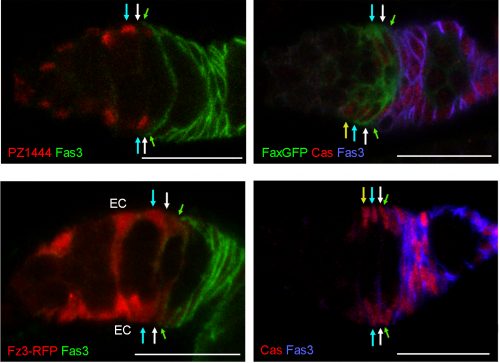
Heterogeneity of FSCs
We found graded cell division rates and graded marker expression across FSC layers. Most important, we found that recent FC production was tightly correlated with FSC location in the most posterior layer (layer 1), leading us to postulate that FCs come directly only from layer 1 FSCs. The process of associating irreversibly with a germline cyst passing through the FSC region has never been visualized, so there was no clear prior indication of where an FC is first “specified.” ECs are anterior to FSCs, so it is almost inevitable that they must derive directly from anterior FSCs, and we also observed movement of anterior FSCs to the EC region by live imaging. Thus, the direct precursors of FCs and ECs are in different FSC layers and as long as an FSC lineage is confined to a single layer it will produce only ECs or only FCs, as often observed at short times after FSC marking. At later times, most surviving FSC lineages amplify and occupy more than one layer, followed by production of both ECs and FCs. Those FSC lineages that are then lost will leave a residue of only, generally long-lived, ECs. Movement of FSCs between layers was also inferred more directly by finding the derivatives of single marked cells in more than one layer at a reasonably high frequency even within 3 days.
The heterogeneity of FSCs presents a complication in identifying FSCs by markers because many proteins that are highly expressed in Escort cells are graded in expression across the FSC domain. For example, more anterior FSCs express much higher levels of the Wnt reporter Frizzled3-RFP and the enhancer trap PZ1444 than posterior FSCs. Other markers, such as Castor, are roughly evenly expressed across FSC layers but are also expressed in FCs. We found that only Fax-GFP stood out as being enriched in FSCs compared with ECs or FCs.
Wnt signaling
We had for many years been puzzled as to why FSCs were lost at an enhanced rate when Wnt pathway activity was either eliminated or increased in FSCs. For Hedgehog and JAK-STAT pathways the results were simpler; more activity caused better survival in competition with wild-type neighbors. With our new understanding of FSC organization and behavior we could look more carefully at the consequences of manipulating Wnt pathway activity, and what we found was straightforward and striking. When Wnt pathway activity is eliminated, FSCs of that lineage become heavily biased towards occupying the most posterior FSC layer and produce FCs, with virtually no EC production. On the other hand, excess Wnt signaling forces FSCs at first into the more anterior FSC layers and eventually to become ECs. These results showed that the strength of Wnt signaling influences the A/P location and fates of FSCs.
Similarity to mammalian epithelial stem cells
With the updated organization of FSCs uncovered, we were amazed to find how similar FSC organization is to mammalian intestinal stem cells. Both FSCs and intestinal stem cells give rise to a constant supply of polarized epithelial cells. Similarities include the number of cells per niche, the organization of stem cells in heterogeneous layers, and use of Wnt as a critical niche factor[9]. Mammalian gut stem cells can produce transit amplifying (TA) or quiescent secretory cells, including Paneth cells, which eventually are retained in the crypt while TA cells migrate away. The initial location of stem cell derivatives that lead to these different outcomes are not clearly defined. Hence the spatial cues for differences in signaling pathway activities (Notch and Wnt) involved in deciding outcomes are not clear. FSCs appear to present a slightly different and simpler paradigm that may also inspire enquiry into an analogous organizational principle for gut stem cells. Wnt signaling is graded across the A/P axis of the FSC community. The relative levels of Wnt signaling dictate whether FSCs adopt more posterior positions and yield predominantly FCs (low Wnt pathway activity) or adopt more anterior positions and become ECs (high Wnt pathway activity), in accord with the natural gradient of Wnt pathway activity in this region. The paradigm of different direct differentiation outcomes for a stem cell population depending on stem cell location might apply to mammalian stem cell communities as well.
Amy Reilein and Daniel Kalderon
Department of Biological Sciences, Columbia University, New York, NY
References
Posted by the Node, on 15 June 2017
Last year we were approached by Andreas Prokop of The University of Manchester (who is also Communications Officer of the British Society for Developmental Biology),  with an offer to write a paper on our experiences of running the Node and using social media to build scientific networks. We – that is Aidan Maartens (the Node’s Community Manager), Catarina Vicente (who previously held the post) and Katherine Brown (Development’s Executive Editor) – accepted, seeing it as a great opportunity to promote our work as well as explore what we have learned after six years. The paper is part of an exciting upcoming Special Issue of Seminars in Cell & Developmental Biology on science communication, and features input from individuals and organisations who use social media
with an offer to write a paper on our experiences of running the Node and using social media to build scientific networks. We – that is Aidan Maartens (the Node’s Community Manager), Catarina Vicente (who previously held the post) and Katherine Brown (Development’s Executive Editor) – accepted, seeing it as a great opportunity to promote our work as well as explore what we have learned after six years. The paper is part of an exciting upcoming Special Issue of Seminars in Cell & Developmental Biology on science communication, and features input from individuals and organisations who use social media
The following excerpts give a taste of where we came from in the article, and you can find a link to it below.
Established in 1953 and initially known as the Journal of Embryology and Experimental Morphology, Development (www.dev.biologists.com) is a leading research journal in developmental biology. Run by the not-for-profit publisher The Company of Biologists, whose mission is to support and inspire the biological community, it has a specific remit to support the needs of developmental biologists. In 2009, a survey conducted by the journal highlighted the idea that Development − seen as a community journal − should be doing more for the community. Specifically, the survey identified a desire for an environment where members of this and related fields (most notably stem cell biology, but also other intersecting fields such as cell biology, evolution and genetics) could gather and interact online, bypassing the need for each internet-savvy researcher to build their own network from scratch. Development responded in 2010 by establishing an online hub called the Node. The site’s name reflects its aim: from a technical perspective, a node is simply a connection point, while developmental biologists know the node as an important group of cells that instruct and organise the activity of others in the early embryo. The Node was hence conceived as an online connection point for developmental biologists. It would provide a place where ideas could be discussed and exchanged by the whole community, without the restrictions of more formal publications, and would encourage an informal style and varied content, as well as dedicated pages for job opportunities and events useful for community members. Importantly, the Node would be open to anyone interested in contributing and would be easy to use. Thus, to some extent, the Node could be considered an online (and hence more flexible and accessible) version of a scientific newsletter − an informal form of communication aimed at a defined group of researchers with a remit to facilitate exchange of ideas and provide information on useful resources.
The Node was conceived as an online connection point for developmental biologists
The digital age has opened the doors to a brave new world of communication outside traditional restrictions of geography, funding and editorial control. While the scientific community in general has not necessarily been quick to adopt and exploit all the opportunities now available, these technologies are changing the way we communicate. The Node, born out of a desire among the developmental biology community for an online communication hub, exemplifies both the opportunities and the challenges of community building online. Perhaps the biggest lesson we have learned in the 6 years of operating the Node is that the initial concept of a self-sustaining community site was unrealistic: the Node relies on ongoing financial, technical and strategic support from The Company of Biologists. However, we have also learned that this support is recognised and appreciated: most members of the developmental biology community are now aware of the Node, even if they don’t actively participate in it, and value its utility as an online hub for the field. Social media, particularly Twitter, have helped us reach a wider audience, and better gather and disseminate information. The original vision of the Node – as your online coffee break, a place to catch up on the latest news from the field − has been realised, although we continue to develop and grow. Currently, significant efforts are focussed on improving the resources section of the site, to help with teaching, advocacy and outreach activities, as well as on growing our reader- and authorship in particular geographic and scientific areas where we are less well represented than we would like. We are also exploring formats that can better facilitate online discussion.
The Node exemplifies both the opportunities and the challenges of community building online
You can read the full paper freely here:
Seminars in Cell & Developmental Biology
https://doi.org/10.1016/j.semcdb.2017.05.009
Posted by Noami Dayan, on 14 June 2017
Closing Date: 15 March 2021
The offered position will provide bioinformatics support to our scientists, with focus on planning, processing, and analysing transcriptomic and epigenomic next-generation sequencing (NGS) data.
Posted by Guillermo Oliver, on 12 June 2017
Closing Date: 15 March 2021
POSTDOCTORAL POSITION is available to study different aspects of lymphatic vasculature development and metabolism in health and disease. Some of the projects include the characterization of the cellular and molecular mechanisms controlling the development of the lymphatic vasculature, endothelial cell plasticity and reprogramming, as well as different aspects related to lymphatic function and metabolism. Highly motivated individuals who recently obtained a PhD. or MD degree and have a strong background in mammalian vascular, molecular and developmental biology are encouraged to apply. Interested individuals should send their curriculum vitae, a brief description of their research interests, and the names of three references to:
Guillermo Oliver, Ph.D
Thomas D Spies Professor of Lymphatic Metabolism
Director Center for Vascular and Developmental Biology
Northwestern University Feinberg School of Medicine
303 East Superior Street, 10-107
Chicago, Illinois 60611
Posted by Paul Conduit, on 12 June 2017
Closing Date: 15 March 2021
A Postdoctoral Research Associate position is available in the Conduit lab to study the role of the nuclear envelope in centrosome assembly. Our lab studies how microtubule formation is regulated in cells, including how microtubule organising centres (MTOCs) are assembled. The post-holder will use Drosophila melanogaster to follow up on our recent discover that the nuclear envelope may help regulate centrosome assembly. They will use CRISPR/HDR to generate a series of new fly strains, and then use advanced live-cell fluorescence imaging, including super-resolution imaging, and biochemistry to establish how the nuclear envelope helps regulate centrosome assembly.
The appointment will be for a period of up to three years starting 2nd October 2017, or as soon as possible thereafter.
Candidates are expected to have (or soon have) a PhD in cell/developmental biology and have experience in fluorescent microscopy. Prior experience in Drosophila, centrosome biology, molecular biology and/or basic biochemistry would be an advantage, but not essential.
Applications should include a C.V. and a brief statement of your scientific background and why you would like to join the lab.
To apply, please follow this link: http://www.jobs.cam.ac.uk/job/13999/
Informal enquiries are welcomed and can be addressed to Dr. Paul Conduit ptc29@cam.ac.uk
Please see the lab website for more information on our research http://conduitlab.zoo.cam.ac.uk