Closing Date: 15 March 2021

The MRC Weatherall Institute of Molecular Medicine (WIMM) has fully funded 4-year Prize PhD (DPhil) Studentships available to start in October 2018. These Studentships are open to outstanding students of any nationality who wish to train in experimental and/or computational biology.
The Institute is a world leading molecular and cell biology centre that focuses on research with application to human disease. It includes the recently opened MRC WIMM Centre for Computational Biology and houses over 500 research and support staff in 50 research groups working on a range of fields in Haematology, Gene Regulation & Epigenetics, Stem Cell Biology, Computational Biology, Cancer Biology, Human Genetics, Infection & Immunity. The Institute is committed to training the next generation of scientists in these fields through its Prize PhD Studentship Programme.
The fully funded studentships include a stipend of £18,000 per annum and cover University and College fees.
Further information on the studentships, how to apply, and the projects available can be found at:
http://www.imm.ox.ac.uk/wimm-prize-studentships-2018
Closing date for submission of applications: Monday, 8 January 2018, 12 noon UK time.
Interviews will take place the week commencing 22 January 2018.
Pure Computational Biology Project Leaders
Hashem Koohy – Machine-learning in gene function, transcription regulation and immunology
Ed Morrissey – Quantitative biology of cell fate
Aleksandr Sahakyan – Regulatory chromosomal domains and genome architecture
Supat Thongjuea – Computational biology of single-cell transcription and gene regulation
Molecular and Cell Biology Project Leaders
Ahmed Ahmed – Experimental therapeutics
Chris Babbs – Causes of congenital anaemia
Oliver Bannard – B cell biology
Andrew Blackford – DNA damage and disease
Walter Bodmer – Colorectal cancer, stem cells, differentiation & drug response
Marella De Bruijn – Developmental haematopoiesis
Zam Cader – Stem cell neurological disease models
Vincenzo Cerundolo – Tumour immunology, vaccine strategies
David Clynes – DNA damage, repair and cancer
Simon Davis – T-cell biology
Hal Drakesmith – Iron and infection
Christian Eggeling – Super-resolution microscopy in immunology
Ben Fairfax – Inflammation, genetics and cancer therapeutics
Marco Fritzsche – Biophysical immunology
Lars Fugger – Multiple sclerosis
Tudor Fulga – MicroRNAs in development and disease
Richard Gibbons – Chromatin, epigenetics & transcription
Anne Goriely – De novo mutations and human disease
Doug Higgs – Gene regulation and epigenetics
Ling-Pei Ho – Lung immunology
Georg Hollander – T cell development and thymus organogenesis
David Jackson – Lymphatic trafficking in inflammation and cancer
Peter McHugh – DNA repair
Adam Mead – Normal and leukaemic haematopoietic stem cell biology
Claus Nerlov – Tissue stem cell genetics
Graham Ogg – Translational skin research
Catherine Porcher – Transcription factors and blood development
Jan Rehwinkel – Innate detection of viruses
Irene Roberts – Trisomy 21, haematopoiesis and leukaemia
Tatjana Sauka-Spengler – Neural crest gene regulatory networks
Alison Simmons – Innate immunity & Crohn’s disease
Alain Townsend – Influenza and ebola, vaccination and treatment
Paresh Vyas – Leukaemic stem cells
Andrew Wilkie – Sperm and craniofacial mutations

 (No Ratings Yet)
(No Ratings Yet)
 Loading...
Loading...


 (No Ratings Yet)
(No Ratings Yet)
 (2 votes)
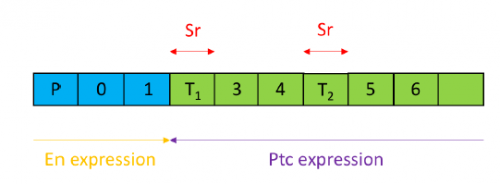
(2 votes) The tectorial membrane (TM) is an extracellular matrix (ECM) that overlies the organ of Corti in the inner ear and is crucial for our sense of hearing. It is composed of collagen fibrils embedded in a tectorin-based matrix. The precise alignment of the collagen fibrils across the TM is a feature considered critical for hearing, but very little is known about how this pattern is generated. On p.
The tectorial membrane (TM) is an extracellular matrix (ECM) that overlies the organ of Corti in the inner ear and is crucial for our sense of hearing. It is composed of collagen fibrils embedded in a tectorin-based matrix. The precise alignment of the collagen fibrils across the TM is a feature considered critical for hearing, but very little is known about how this pattern is generated. On p.  Embryonic patterning is dependent on the establishment of the anteroposterior (AP) and dorsoventral axes early in development. In mammalian embryos, these axes are established by a breaking of symmetry in the epiblast, which involves signals from the extra-embryonic tissues. However, the molecular mechanisms that control this process are still not fully understood. On p.
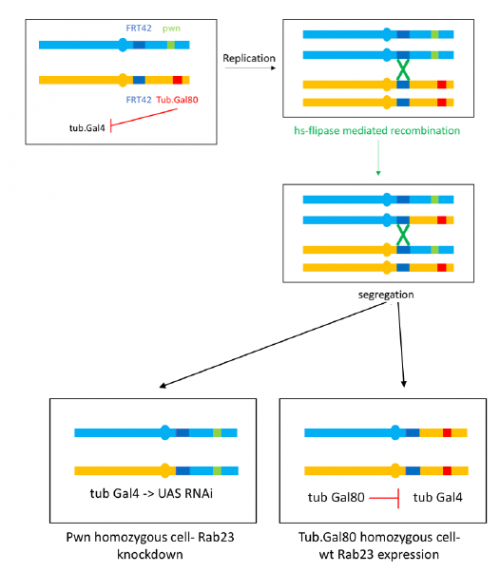
Embryonic patterning is dependent on the establishment of the anteroposterior (AP) and dorsoventral axes early in development. In mammalian embryos, these axes are established by a breaking of symmetry in the epiblast, which involves signals from the extra-embryonic tissues. However, the molecular mechanisms that control this process are still not fully understood. On p.  The core autophagy protein Atg16L1 has been identified as a genetic risk factor in inflammatory bowel disease, but how it plays this role has remained unclear. On p.
The core autophagy protein Atg16L1 has been identified as a genetic risk factor in inflammatory bowel disease, but how it plays this role has remained unclear. On p.  Christiane Nüsslein-Volhard is Director Emeritus at the Max Planck Institute for Developmental Biology in Tübingen, Germany. In 1995, she was awarded the Nobel Prize for Physiology and Medicine, along with Eric Wieschaus and Edward Lewis, for her work on the genetic control of embryogenesis using the fruit fly Drosophila melanogaster. In the 1990s, she transitioned her lab to working with zebrafish (Danio rerio), using similar forward genetic approaches to those that had proved so successful in Drosophila to uncover key regulators of vertebrate development. We met with Christiane at the recent International Society for Developmental Biology (ISDB) meeting in Singapore, to talk about her research, the impact of the Nobel Prize and the challenges of being a ‘woman in science’. See the
Christiane Nüsslein-Volhard is Director Emeritus at the Max Planck Institute for Developmental Biology in Tübingen, Germany. In 1995, she was awarded the Nobel Prize for Physiology and Medicine, along with Eric Wieschaus and Edward Lewis, for her work on the genetic control of embryogenesis using the fruit fly Drosophila melanogaster. In the 1990s, she transitioned her lab to working with zebrafish (Danio rerio), using similar forward genetic approaches to those that had proved so successful in Drosophila to uncover key regulators of vertebrate development. We met with Christiane at the recent International Society for Developmental Biology (ISDB) meeting in Singapore, to talk about her research, the impact of the Nobel Prize and the challenges of being a ‘woman in science’. See the  During development, genes are transcribed at specific times, locations and levels. In recent years, the emergence of quantitative tools has significantly advanced our ability to measure transcription with high spatiotemporal resolution in vivo. Here, Angela DePace and co-workers highlight recent studies that have used these tools to characterize transcription during development, and discuss the mechanisms that contribute to the precision and accuracy of the timing, location and level of transcription. See the
During development, genes are transcribed at specific times, locations and levels. In recent years, the emergence of quantitative tools has significantly advanced our ability to measure transcription with high spatiotemporal resolution in vivo. Here, Angela DePace and co-workers highlight recent studies that have used these tools to characterize transcription during development, and discuss the mechanisms that contribute to the precision and accuracy of the timing, location and level of transcription. See the  Cortical interneurons are a diverse group of neurons that project locally and are crucial for regulating information processing and flow throughout the cortex. Recent studies in mice have advanced our understanding of how these neurons are specified, migrate and mature. Here, John Rubenstein and colleagues evaluate new findings that provide insights into the development of cortical interneurons and that shed light on when their fate is determined, on the influence that regional domains have on their development, and on the role that key transcription factors and other crucial regulatory genes play in these events. See the
Cortical interneurons are a diverse group of neurons that project locally and are crucial for regulating information processing and flow throughout the cortex. Recent studies in mice have advanced our understanding of how these neurons are specified, migrate and mature. Here, John Rubenstein and colleagues evaluate new findings that provide insights into the development of cortical interneurons and that shed light on when their fate is determined, on the influence that regional domains have on their development, and on the role that key transcription factors and other crucial regulatory genes play in these events. See the  The 2nd NEUBIAS school for Bioimage Analysts will be organized in Jan. 2018 in Szeged, Hungary, and the registration is now open (Organizers: Jean-Yves Tinevez & Kota Miura). Please visit the linked URL below for more details. This school is the most advanced among three levels of NEUBIAS school. Deadline for the registration is Nov. 9th.
The 2nd NEUBIAS school for Bioimage Analysts will be organized in Jan. 2018 in Szeged, Hungary, and the registration is now open (Organizers: Jean-Yves Tinevez & Kota Miura). Please visit the linked URL below for more details. This school is the most advanced among three levels of NEUBIAS school. Deadline for the registration is Nov. 9th.