Public Engagement Officer
Posted by stemcellsjobs, on 10 July 2014
Closing Date: 15 March 2021
Department/Location: Wellcome Trust – Medical Research Council Cambridge Stem Cell Institute, University of Cambridge
Salary: £28,132-£36,661
Reference: PS03788
Closing date: 10 August 2014
Fixed-term: The funds for this post are available until 30 June 2017 in the first instance.
The Stem Cell Institute is a world-leading centre of excellence in stem cell biology and regenerative medicine, supported by a strategic funding partnership between the Wellcome Trust and the Medical Research Council (www.stemcells.cam.ac.uk).
Increasing awareness and understanding of the promise and challenges of stem cell research is embedded in the Stem Cell Institute vision; “deep understanding of stem cell biology for the prevention and treatment of human disease”. The Institute’s 5 strategic goals include “Communication and public engagement; providing reliable information, useful resources, and dialogue opportunities for a range of audiences including schools, policy makers, patient groups, professional bodies and the media”.
The central role of the Public Engagement Officer (PEO) is to foster a community of scientists who recognise the importance of dialogue with the public and who have the skills and opportunities to undertake public engagement activities. The PEO will build networks with local schools, arts groups and businesses to develop activities/events with/for them. The PEO will liaise with other Public Engagement officers in the University, Wellcome Trust Centres and Medical Research Council Institutes/Units and will participate in international networks via EuroStemCell, ISSCR and other trans-national initiatives.
We are seeking a dynamic, innovative and self-motivated individual who will bring expertise and leadership for the Institute’s outreach activities. Experience of working in the HE or research sector would be an advantage. Educated to degree level (or equivalent) in a scientific discipline, you will be responsible for growing a community of researchers who are enthusiastic about dialogue with the public and who have the skills and confidence to deliver stimulating interactive events.
The Stem Cell Institute, is currently spread across several sites in Cambridge, you will organise training, logistics and activities with the aim that all groups in SCI will contribute to at least one event each year. The PEO will expand the existing Public Engagement strategy and design and implement innovative public engagement activities that will interconnect the SCI with target audiences. You will develop measures for evaluating the effectiveness and impact of public engagement activities. You will submit reports to the Institute steering committee and sponsors and will prepare funding applications for public engagement activities.
You must demonstrate a proven track record in relationship building, event organisation, report writing, and data management. You will have outstanding organisational and administrative experience and be comfortable working to tight deadlines with minimal supervision. You should have demonstrable experience in web-based/social media communication and you should have excellent written and verbal communication and negotiation skills. You will be IT literate and able to work both on your own and as part of a team.
The post will report to the Institute Director. You will have a degree (or equivalent).
Once an offer of employment has been accepted, the successful candidate will be required to undergo a health assessment.
To apply online for this vacancy and to view further information about the role, please visit: http://www.jobs.cam.ac.uk/job/4403. This will take you to the role on the University’s Job Opportunities pages. There you will need to click on the ‘Apply online’ button and register an account with the University’s Web Recruitment System (if you have not already) and log in before completing the online application form.
Please upload your current CV and cover letter with your application by Sunday 10th August 2014.
Informal enquiries are also welcome via email: cscrjobs@cscr.cam.ac.uk.
Interviews will be held towards the end of August 2014. If you have not been invited for interview by 18th August 2014, you have not been successful on this occasion.
Please quote reference PS03788 on your application and in any correspondence about this vacancy.
The University values diversity and is committed to equality of opportunity.
The University has a responsibility to ensure that all employees are eligible to live and work in the UK.


 (No Ratings Yet)
(No Ratings Yet)
 (1 votes)
(1 votes)


 Embryonic stem cell (ESC) cultures display a marked heterogeneity in the expression of Nanog, one of several core pluripotency factors required for proper development in vivo. In addition, Nanog levels have also been shown to fluctuate in individual ESCs in vitro; however, the extent and functional consequences of these fluctuations in different pluripotency states has not been fully established. Now, on p.
Embryonic stem cell (ESC) cultures display a marked heterogeneity in the expression of Nanog, one of several core pluripotency factors required for proper development in vivo. In addition, Nanog levels have also been shown to fluctuate in individual ESCs in vitro; however, the extent and functional consequences of these fluctuations in different pluripotency states has not been fully established. Now, on p.  The balance between excitatory versus inhibitory neuron specification during development is crucial for sensory information processing in later life. The basic helix-loop-helix (bHLH) transcription factors Ascl1 and Ptf1a are crucial for establishing this specificity in the dorsal spinal cord, but how these two factors, which recognise a similiar DNA motif, can have opposite downstream effects is unclear. Now, on p.
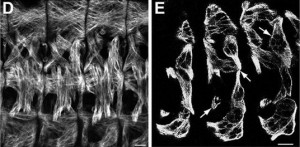
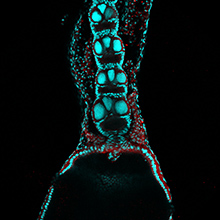
The balance between excitatory versus inhibitory neuron specification during development is crucial for sensory information processing in later life. The basic helix-loop-helix (bHLH) transcription factors Ascl1 and Ptf1a are crucial for establishing this specificity in the dorsal spinal cord, but how these two factors, which recognise a similiar DNA motif, can have opposite downstream effects is unclear. Now, on p.  During blastocyst development, asymmetric cell divisions generate polar and apolar daughter cells, which organise into outer and inner positions, respectively, to form the trophectoderm (TE) and inner cell mass (ICM) lineages. The Hippo signaling pathway is crucial for setting up this early lineage specification, but how Hippo signaling relates to cell position and polarity remains unclear. In this issue (p.
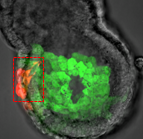
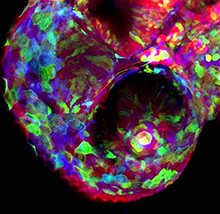
During blastocyst development, asymmetric cell divisions generate polar and apolar daughter cells, which organise into outer and inner positions, respectively, to form the trophectoderm (TE) and inner cell mass (ICM) lineages. The Hippo signaling pathway is crucial for setting up this early lineage specification, but how Hippo signaling relates to cell position and polarity remains unclear. In this issue (p.  Cell polarity is fundamental for biological activity across many varied cell types within different animal species. Intracellular trafficking regulates the differential distribution of proteins that is fundamental to establishing cell polarity, but how cell polarity regulators exert their effects on trafficking machinery is largely unknown. Now, on p.
Cell polarity is fundamental for biological activity across many varied cell types within different animal species. Intracellular trafficking regulates the differential distribution of proteins that is fundamental to establishing cell polarity, but how cell polarity regulators exert their effects on trafficking machinery is largely unknown. Now, on p.  In March 2014, the RIKEN Center for Developmental Biology in Kobe, Japan, hosted a meeting entitled ‘Regeneration of Organs: Programming and Self-Organization’. Scientists from across the globe met to discuss current research on regeneration, organ morphogenesis and self-organization – and the links between these fields. As discussed by Daniel Goldman, a diverse range of experimental models and organ systems was presented at the meeting, and the speakers aptly illustrated the unique power of each. See the Meeting Review on p
In March 2014, the RIKEN Center for Developmental Biology in Kobe, Japan, hosted a meeting entitled ‘Regeneration of Organs: Programming and Self-Organization’. Scientists from across the globe met to discuss current research on regeneration, organ morphogenesis and self-organization – and the links between these fields. As discussed by Daniel Goldman, a diverse range of experimental models and organ systems was presented at the meeting, and the speakers aptly illustrated the unique power of each. See the Meeting Review on p 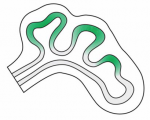 Branching morphogenesis is the developmental program that builds the epithelial trees of various organs, including the airways of the lung, the collecting ducts of the kidney, and the ducts of the mammary and salivary glands. R
Branching morphogenesis is the developmental program that builds the epithelial trees of various organs, including the airways of the lung, the collecting ducts of the kidney, and the ducts of the mammary and salivary glands. R The formation of the vasculature is essential for tissue maintenance and regeneration, and understanding how vascular formation is coordinated in vivo can offer valuable insights into engineering approaches for therapeutic vascularization and angiogenesis. Here, Kyung Min Park and Sharon Gerecht discuss how the process of vascular development can be used to guide approaches to engineering vasculature. See the Review on p.
The formation of the vasculature is essential for tissue maintenance and regeneration, and understanding how vascular formation is coordinated in vivo can offer valuable insights into engineering approaches for therapeutic vascularization and angiogenesis. Here, Kyung Min Park and Sharon Gerecht discuss how the process of vascular development can be used to guide approaches to engineering vasculature. See the Review on p.