An interview with Cheryll Tickle
Posted by the Node, on 5 April 2016
This interview first featured in Development.
Cheryll Tickle is an Emeritus Professor at the University of Bath, UK. She dedicated her long research career mainly to the study of limb development in the chick, and has received numerous awards for her contributions to science, including being elected a Fellow of The Royal Society and receiving a CBE from the Queen. This year the British Society for Developmental Biology (BSDB) has created a new award in her honour, the Cheryll Tickle Medal, to be awarded to a mid-career, female scientist for outstanding achievements in the field. We asked Cheryll what this award means to her and how science has changed during her career.
 How did you first become interested in developmental biology and why did you decide to study limb development?
How did you first become interested in developmental biology and why did you decide to study limb development?
As an undergraduate I was most interested in cell biology. I was excited by the idea of understanding how cells behave and how they build tissues in embryos. At the time, developmental biology was usually called embryology, and it was the only subject that seemed totally mysterious and unfathomable: there were no general principles that I could get hold of. So I did a PhD in cell biology in the lab of Adam Curtis, who had just become Professor at Glasgow in the first Department of Cell Biology in the UK. I studied a phenomenon known as ‘cell sorting’, which was very exciting in the 1960s. Malcolm Steinberg, building on earlier work in amphibians, had shown that if you disaggregated cells from the various tissues of the embryonic chick and mixed them together, they would sort themselves out so that cells of the same type preferentially adhered to each other. It was not clear whether a similar process happened in embryos, but it was one possible way by which you could build patterns of differentiated cells.
One of the cell types I used during my PhD in Glasgow was limb bud cells. In the limb you could imagine how cells might get committed at random to become cartilage cells and then ‘sort out’ to the centre of the limb. At that time Donald Ede, who was based in Edinburgh, used to come over to Glasgow with his PhD student Oliver Flint to use Adam’s Couette viscometer to measure cell adhesiveness. Donald was looking at limb bud development in normal chick embryos and in a mutant called talpid3, which was polydactylous and had various defects. His research further reinforced my idea to study limb development and ignited an interest in the mutant. After Donald retired, Dave Burt at the Roslin Institute took on the maintenance of the talpid3 mutant flock. We continued to study it and eventually identified the gene responsible for the phenotype and showed that it is a ciliopathy. Very recently, several groups have identified mutations in the talpid3 gene in patients with Joubert syndrome.
After my PhD I was awarded a NATO fellowship to do a postdoc in America, and I worked with John Trinkaus in Yale for 2 years, on aggregation of cell lines and cell behaviour in early fish embryo development. However, when I went for my fellowship interview I was asked what I would eventually like to work on. When I said ‘limb development’, the person who interviewed me said that he wouldn’t recommend that because everything was already known!
Did any mentors play an important role in your career?
There were three important mentors in my career. I was very fortunate with my PhD supervisor Adam Curtis, my first postdoctoral supervisor John Trinkaus and then of course with Lewis Wolpert, with whom I did a second postdoc funded by the MRC. They all supported me. I was recently asked why it was that a group of successful women embryologists came through from the 1960s and 1970s. I think in my case it was partially the attitude of the men I worked with. They allowed me the freedom to develop and did not put me down.
Another person that was very influential was Bruce Alberts, who I got to work with when he did a sabbatical in the Wolpert lab. He came armed with all these pots of beads and we started using the beads as carriers for applying chemicals to the limb bud. This technique is widely used now, but it was really being developed at the time. We used it to apply retinoic acid to the wing bud. Brigid Hogan was also very supportive during my early career when she was in the UK. Even though I wasn’t in the mouse field, she was supportive, encouraging me to apply for grants and so on.
How do you think that science has changed over the years in which you have been a researcher?
I think people tend to forget that in the 1960s and 1970s there was a lot of emphasis on cell biology, within the confines of what was possible. Quite a lot of the work I did was looking at cells in the limb, their morphology, whether they had gap junctions or not, etc. There were also quite a lot of productive interactions between people in different fields, particularly with mathematicians. During my PhD I collaborated with a statistician, Rob Elton, and we carried out a nearest neighbour analysis to quantitate the degree of sorting out in the aggregates that I had made. Computer modelling was also being used. Donald Ede had made some computer models of limb development, and I believe the computer that he used took up an entire room! These approaches bringing cell biology and/or mathematical modelling to bear on problems in embryonic development are now very much at the forefront of the field. Since my early work on limb development there have been two revolutions: being able to understand the genetic basis of development and the ability to do genome-wide analysis.
When I was carrying out my PhD, there were not so many people doing science, and the funding was easier to obtain. There wasn’t the level of competitiveness that you can see now, although it was very competitive in some areas! We seemed to have a lot more time to think and to ‘undertake scholarship’, such as studying primary papers in detail rather than just reading a review.
What do you think are the exciting scientific questions at the moment?
I think real progress is being made in the area of evo-devo, particularly in this new age of genomics. We are on the brink of learning a lot. There will be a huge amount of data, and for a while it will be very confusing. A little like the Hox gene clusters when they were first discovered. Eventually though it will all become clear. I think in general that developmental biology has not yet reaped the full rewards of the genomic age.
You are now an Emeritus Professor at Bath University. Do you miss doing science?
I really enjoyed doing science. When you discover something there is a real buzz. After a seminar in Switzerland, a member of the audience said that he had enjoyed hearing all about my tedious experiments! But of course I didn’t find them tedious. Doing experiments on chick embryos, cutting bits out, grafting them, observing them develop was always fascinating. So yes, I miss it a lot. No one gives you advice on how to finish your research career! But I am still very busy writing reviews, sitting on committees, and so on.
Over the years you have received many awards, including being elected a fellow of The Royal Society and being the first awardee of the Waddington Medal. What do these awards mean to you?
Receiving these awards is a bit overwhelming, but of course very nice. I remember that when I got the letter about the Waddington Medal I couldn’t believe it! Being a fellow of The Royal Society has been very positive. The Society is very supportive and you have the opportunity to meet people working on different areas of science, which is very interesting. Later on I also became a Royal Society Professor, so the Society directly supported my research, for which of course I am extremely grateful.
I think I received these awards because I was in the right place at the right time. I was lucky in my choice of research field and to work so closely with Lewis Wolpert for many years. The other important thing was the large number of collaborations I established with outstanding scientists in the field. I could combine my expertise with that of people like Denis Duboule and Gail Martin, working with Juan-Carlos Izpisua-Belmonte and Lee Niswander who were doing postdocs in their respective labs at that time. At UCL, I also worked with Anne Warner and Jon Clarke. By pooling our expertise, we were able to do a lot more than I would have been able to do on my own. And of course I had fantastic undergraduates, PhD students and postdocs. I feel that these awards were not just for me but for the whole enterprise.
This year the BSDB created a new award in your honour, the Cheryll Tickle Medal, which will be awarded to Abigail Tucker from King’s College London. What does it mean for you to have a medal named after you?
It is very humbling and a huge honour. I am actually going to present the award to Abigail. I was not involved in the selection of the winner, but I think Abigail is a very worthy recipient. I am very familiar with her work, in particular on how teeth are positioned within the jaw, which very much mirrors some of the work I did on the limb. It is research of fundamental importance, not just for our understanding of tooth development but also more generally.
This medal is awarded to a mid-career, female scientist for her outstanding achievements in the field of developmental biology. Do you think that there is the need for awards that specifically recognise the contributions of women in science?
The Chair of the BSDB, Ottoline Leyser, wrote in a recent society newsletter that all young scientists need encouragement and support, both men and women. However, because there is such a huge attrition in the number of women who go on to embark upon a scientific career, we need to celebrate the success of women and what they achieve. I used to feel that you could sum up the situation of women in science by saying that they are generally undervalued, overlooked and ignored. Fortunately things are changing – although it has been very slow.
There are many types of barriers for women in research. Having children is obviously a big barrier, but not being encouraged, especially if you are in a total male environment, can be a bit demoralising. While I was supported by my mentor, during my PhD in Glasgow I was the only female PhD student in the department. But when I went to Yale there were lots of women students around, and that made a quite a difference. I have tried to encourage other women, but maybe I have not been highly visible. I think that if every woman did something on a personal level we could easily conquer a lot of these difficulties. But of course we need to support our male colleagues as well, particularly at the beginning of their careers.
You said before (in an article in Nature) that current PhD students are not allowed as much freedom or to make as many mistakes as you did during your graduate training. Why do you think this is?
I think this is related to supervisors being too hands-on. I personally think that students should have more chances to make mistakes. In order to learn how to skate you need to fall over, and it is the same with science. You need to make some gaffes and learn from them. However, it is so competitive to get grants that there is increasing pressure to get results and not waste money. There is also more emphasis on training in narrow areas, rather than encouraging creativity. You are constrained to be in a particular way and receive particular kinds of training. It is all a bit too prescribed.
What is your advice for young scientists?
My advice would be to study something that you are really interested in. That is the most important thing. You are going to have to work extremely hard, and there will be quite a lot of lows as well as highs. It doesn’t really matter what the topic is, but it must be personally extremely interesting. I think a key attribute of a successful scientist is doggedness. Also, I would advise you to find people with whom you can work and share expertise. That is the way to make real progress.
You have been a director of The Company of Biologists for several years. How do you see the role of The Company of Biologists in the scientific community?
The Company of Biologists is a charity and not-for-profit publishing company, originally created in 1925 by the zoologist George Parker Bidder III to rescue the British Journal of Experimental Biology, which was failing. It now publishes a portfolio of journals, including Development, and has the aim of supporting research across all branches of biology, including the developmental biology field. I think The Company does not receive enough recognition for what it does. It gives huge amounts of money to the BSDB – both block grants to support its meetings but also travel grants. The Company of Biologists also awards its own travel grants and runs workshops. Sometimes you see a meeting that says ‘supported by The Company of Biologists’ along with many other sponsors, but people don’t really realise how much The Company gives to the field.
What would people be surprised to find out about you?
You could say that I have shared a stage with Bob Dylan. I was awarded an honorary degree from St Andrews University at the same time as Bob Dylan. There was an article in the New Musical Express that had a picture of me standing across the room from him, looking very pleased. One of my ex-students, Sarah Wedden, said that my street cred went up in the eyes of her teenage son!


 (2 votes)
(2 votes)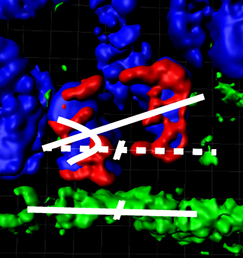 Mutations affecting tubulin genes have been implicated in a range of human neurological disorders but very little is known about the cellular mechanisms that underlie these disorders. Now, on p.
Mutations affecting tubulin genes have been implicated in a range of human neurological disorders but very little is known about the cellular mechanisms that underlie these disorders. Now, on p. 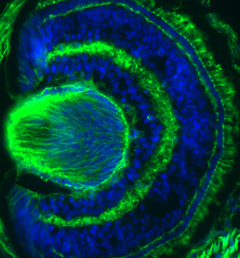
 The liver is a vital organ, and understanding how it develops can provide key insights into liver disorders and regeneration. Here, Mary Weiss, Margaret Buckingham and colleagues use a retrospective clonal approach to provide an in-depth analysis of cell behaviour during liver development in mice (p.
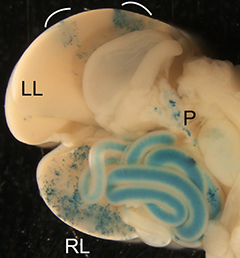
The liver is a vital organ, and understanding how it develops can provide key insights into liver disorders and regeneration. Here, Mary Weiss, Margaret Buckingham and colleagues use a retrospective clonal approach to provide an in-depth analysis of cell behaviour during liver development in mice (p. 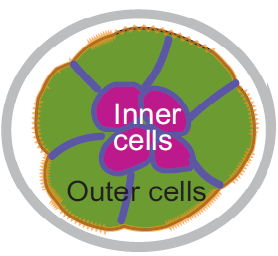 Chazaud and Yamanaka discuss recent advances in live imaging, computational modelling and single cell analyses that provide insights into how the first three cell lineages of the mouse embryo are generated.
Chazaud and Yamanaka discuss recent advances in live imaging, computational modelling and single cell analyses that provide insights into how the first three cell lineages of the mouse embryo are generated.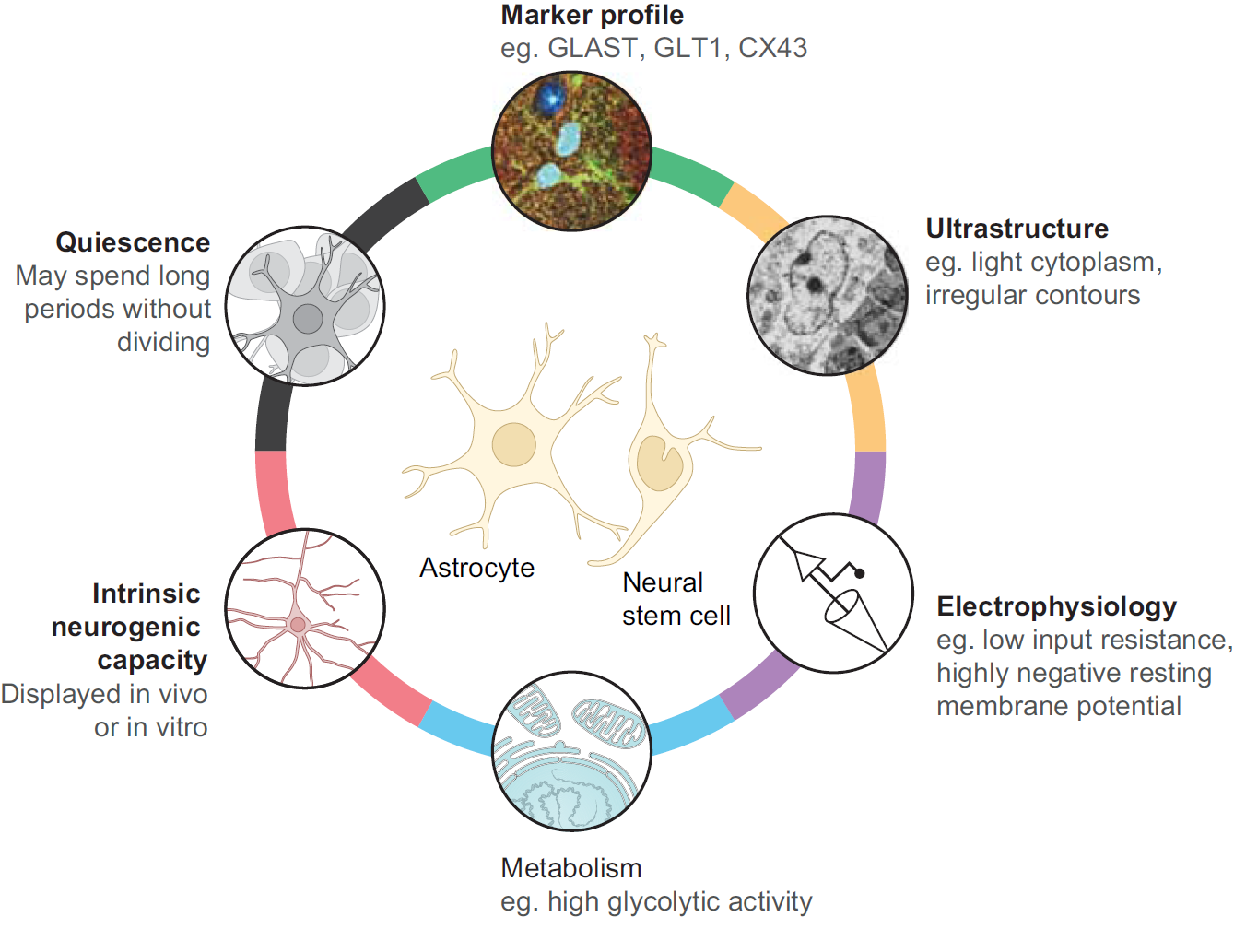 Unlocking the neurogenic potential of astrocytes in different brain regions
Unlocking the neurogenic potential of astrocytes in different brain regions
 (No Ratings Yet)
(No Ratings Yet) Following Development’s call for papers for their upcoming
Following Development’s call for papers for their upcoming  – Liangyu discussed how he adapted the auxin-inducible degradation system to C. elegans, providing a new tool for
– Liangyu discussed how he adapted the auxin-inducible degradation system to C. elegans, providing a new tool for  Also on the Node
Also on the Node
