June in preprints
Posted by the Node, on 10 July 2024
Welcome to our monthly trawl for developmental and stem cell biology (and related) preprints.
The preprints this month are hosted on bioRxiv and arXiv – use these links below to get to the section you want:
- Patterning & signalling
- Morphogenesis & mechanics
- Genes & genomes
- Stem cells, regeneration & disease modelling
- Plant development
- Evo-devo
Developmental biology
| Patterning & signalling
Metabolic control by the Bithorax Complex-Wnt signaling crosstalk in Drosophila
Rajitha-Udakara-Sampath Hemba-Waduge, Mengmeng Liu, Xiao Li, Jasmine L. Sun, Elisabeth A. Budslick, Sarah E. Bondos, Jun-Yuan Ji
Victorio Palacio, Anna Pancho, Angela Morabito, Jonas Malkmus, Zhisong He, Geoffrey Soussi, Rolf Zeller, Barbara Treutlein, Aimée Zuniga
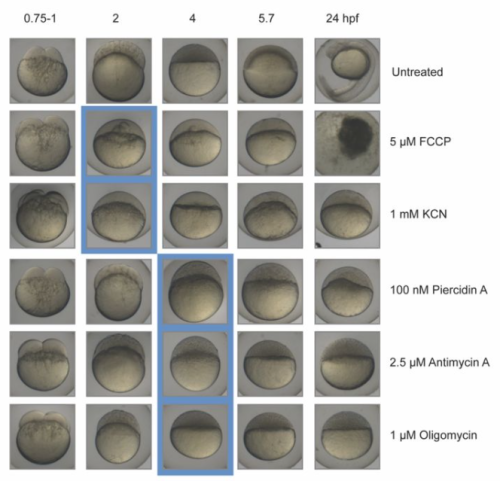
Growth-induced physiological hypoxia correlates with growth deceleration during normal development
Yifan Zhao, Cyrille Alexandre, Gavin Kelly, Gantas Perez-Mockus, Jean-Paul Vincent
Metabolic activities are selective modulators for individual segmentation clock processes
Mitsuhiro Matsuda, Jorge Lázaro, Miki Ebisuya
Li Tong, Faiza Batool, Yueh-Ho Chiu, Yudong Zhou, Xiaolun Ma, Santosh Atanur, Wei Cui
Corine M. van der Weele, Katrina C. Hospes, Katherine E. Rowe, William R. Jeffery
Illuminating morphogen and patterning dynamics with optogenetic control of morphogen production
Dirk Benzinger, James Briscoe
Combinatorial Wnt signaling landscape during brachiopod anteroposterior patterning
Bruno C. Vellutini, José M. Martín-Durán, Aina Børve, Andreas Hejnol
Depolarization induces calcium-dependent BMP4 release from mouse embryonic palate mesenchyme
Mikaela L Follmer, Trevor Isner, Yunus H. Ozekin, Claire Levitt, Emily Anne Bates
Lineage-specific CDK activity dynamics characterize early mammalian development
Bechara Saykali, Andy D. Tran, James A. Cornwell, Matthew A. Caldwell, Paniz Rezvan Sangsari, Nicole Y. Morgan, Michael J. Kruhlak, Steven D. Cappell, Sergio Ruiz
Dachsous and Fat coordinately repress the Dachs-Dlish-Approximated complex to control growth
Hitoshi Matakatsu, Richard G. Fehon
Alison Heffer, Choongheon Lee, Joseph C. Holt, Amy E. Kiernan
Xiaoyang Liu, Mingxi Yu, Tiancheng Wang, Xiangdong Hu, Rui Zhong, Yuan Xiao, Yan Xu, Mei Zhang, Shuang Tang
Decorin enhances metabolic maturation by activating AMPK-PGC1A pathway in cardiac organoids
Myeong-Hwa Song, Seongmin Jun, Seung-Cheol Choi, Ji Eun Na, Im Joo Rhyu, Sun Wook Hwang, Minji Jeon, Do-Sun Lim
Kara A. Nelson, Kari F. Lenhart, Lauren Anllo, Stephen DiNardo
| Morphogenesis & mechanics
Jakub Sumbal, Robin P. Journot, Marisa M. Faraldo, Zuzana Sumbalova Koledova, Silvia Fre
Vasily Borisov, Fedor Shkil
Congenital heart defects differ following left versus right avian cardiac neural crest ablation
Tatiana Solovieva, Marianne E. Bronner
Sara Pietroforte, Makenzie Plough, Farners Amargant
Shuhei So, Masayo Asakawa, Hitoshi Sawa
Control of epiblast cell fate by mechanical cues
Charlène Guillot, Yannis Djeffal, Mattia Serra, Olivier Pourquié
Mechanical Strain Activates Planar Cell Polarity Signaling to Coordinate Vascular Cell Dynamics
Lieke Golbach, Tanumoy Saha, Maria Odenthal-Schnittler, Jenny Lücking, Ana Velic, Emir Bora Akmeric, Dorothee Bornhorst, Oliver Popp, Philipp Mertins, Felix Gunawan, Holger Gerhardt, Boris Macek, Britta Trappmann, Hans J. Schnittler, Milos Galic, Maja Matis
Anastasia Chugunova, Hannah Keresztes, Roksolana Kobylinska, Maria Novatchkova, Thomas Lendl, Marcus Strobl, Michael Schutzbier, Gerhard Dürnberger, Richard Imre, Elisabeth Roitinger, Pawel Pasierbek, Alberto Moreno Cencerrado, Marlene Brandstetter, Thomas Köcher, Benedikt Agerer, Jakob-Wendelin Genger, Andreas Bergthaler, Andrea Pauli
| Genes & genomes
Transcriptomic Analysis of the Spatiotemporal Axis of Oogenesis and Fertilization in C. elegans
Yangqi Su, Jonathan Shea, Darla DeStephanis, Zhengchang Su
Heterochromatin protein ERH represses alternative cell fates during early mammalian differentiation
Andrew Katznelson, Blake Hernandez, Holly Fahning, Jingchao Zhang, Adam Burton, Maria-Elena Torres-Padilla, Nicolas Plachta, Kenneth S. Zaret, Ryan L. McCarthy
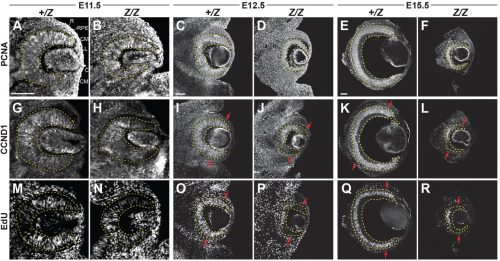
Francesca R. Napoli, Xiaodong Li, Alan A. Hurtado, Edward M. Levine
Shannon H. Carroll, Sogand Schafer, Eileen Dalessandro, Thach-Vu Ho, Yang Chai, Eric C. Liao
The requirement of GW182 in miRNA-mediated gene silencing in Drosophila larval development
Eriko Matsuura-Suzuki, Kori Kiyokawa, Shintaro Iwasaki, Yukihide Tomari
Jordy Dekker, Wendy Lam, Herma C. van der Linde, Floris Ophorst, Charlotte de Konink, Rachel Schot, Gert-Jan Kremers, Leslie E. Sanderson, Woutje M. Berdowski, Geeske M. van Woerden, Grazia M.S. Mancini, Tjakko J. van Ham
Jin Man, Xian Shu, Haoer Shi, Xue Xia, Yusanjiang Abula, Yuu Kimata
Spinal motor neuron development and metabolism are transcriptionally regulated by Nuclear Factor IA
Julia Gauberg, Kevin B. Moreno, Karthik Jayaraman, Sara Abumeri, Sarah Jenkins, Alisa M. Salazar, Hiruy S Meharena, Stacey M Glasgow
RFC1 regulates the expansion of neural progenitors in the developing zebrafish cerebellum
Fanny Nobilleau, Sébastien Audet, Sanaa Turk, Charlotte Zaouter, Meijiang Liao, Nicolas Pilon, Martine Tétreault, Shunmoogum A. Patten, Éric Samarut
| Stem cells, regeneration & disease modelling
Ephrin Forward Signaling Controls Interspecies Cell Competition in Pluripotent Stem Cells
Junichi Tanaka, Yuri Kondo, Masahiro Sakurai, Anri Sawada, Youngmin Hwang, Akihiro Miura, Yuko Shimamura, Dai Shimizu, Yingying Hu, Hemanta Sarmah, Zurab Ninish, James Cai, Jun Wu, Munemasa Mori
Aniket S. Joshi, Micah B. Castillo, Meiricris Tomaz da Silva, Preethi H. Gunaratne, Radbod Darabi, Yu Liu, Ashok Kumar
Gabriela S. Vida, Elizabeth Botto, Stephen DiNardo
Mary-Bronwen L. Chalkley, Lindsey N. Guerin, Tenhir Iyer, Samantha Mallahan, Sydney Nelson, Mustafa Sahin, Emily Hodges, Kevin C. Ess, Rebecca A. Ihrie
Yang Yang, Yinan Zhou, Gary Wessel, Weihua Hu, Dongdong Xu
Inês Caramelo, Vera M. Mendes, Catarina Domingues, Sandra I. Anjo, Margarida Geraldo, Carla M. P. Cardoso, Mário Grãos, Bruno Manadas
Yijian Li, Lingling Ge, Bangqi Ren, Xue Zhang, Zhiyuan Yin, Hongling Liu, Yuli Yang, Yong Liu, Haiwei Xu
Harnessing the regenerative potential of interleukin11 to enhance heart repair
Kwangdeok Shin, Anjelica Rodriguez-Parks, Chanul Kim, Isabella M. Silaban, Yu Xia, Jisheng Sun, Chenyang Dong, Sunduz Keles, Jinhu Wang, Jingli Cao, Junsu Kang
Inhibition of CELA1 Improves Septation in the Mouse Hyperoxia Model of Impaired Alveolar Development
Noah J. Smith, Rashika Joshi, Hitesh Desmukh, Jerilyn Gray, Andrea D. Edwards, Elham Shahreki, Brian M. Varisco
Laura Isabella Arbanas, Emanuel Cura Costa, Osvaldo Chara, Leo Otsuki, Elly Margaret Tanaka
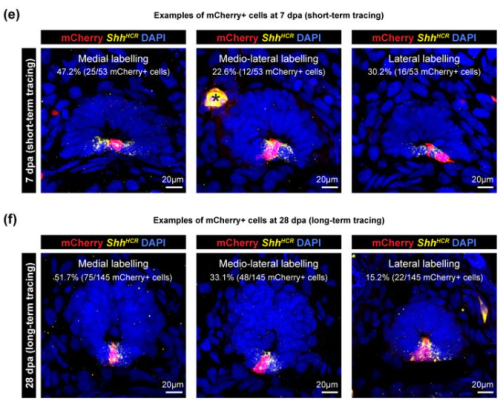
Vivian Jou, Sophia M. Peña, Jessica A. Lehoczky
Ahmed K. Elsayed, Noura Aldous, Nehad Alajez, Essam M. Abdelalim
Stochastic cell-intrinsic stem cell decisions control colony growth in planarians
Tamar Frankovits, Prakash Varkey Cherian, Yarden Yesharim, Simon Dobler, Omri Wurtzel
V. Astro, K. Cardona-Londoño, L.V. Cortés-Medina, R. Alghamdi, G. Ramírez-Calderón, F. Kefalas, J. Dilmé-Capó, S. Radío, A. Adamo
Resolving human α versus β cell fate allocation for the generation of stem cell-derived islets
Melis Akgün Canan, Corinna Cozzitorto, Michael Sterr, Lama Saber, Eunike S.A. Setyono, Xianming Wang, Juliane Merl-Pham, Tobias Greisle, Ingo Burtscher, Heiko Lickert
Fabian Doktor, Rebeca Lopes Figueira, Victoria Fortuna, George Biouss, Kaya Stasiewicz, Mikal Obed, Kasra Khalaj, Lina Antounians, Augusto Zani
Shoya Iwanami, Toshiko Sato, Hiroshi Haeno, Longchen Xu, Keimyo Imamura, Jun Ooehara, Xun Lan, Hiromitsu Nakauchi, Shingo Iwami, Ryo Yamamoto
Wenteng He, Qing Luo, Jian Zhao, Mengting Wang, Luohua Feng, Allan Zhao, Ahmed Reda, Eva Lindgren, Jan-Bernd Strukenborg, Jiayu Chen, Qiaolin Deng
Hepatocyte-derived extracellular vesicles regulate liver regeneration after partial hepatectomy
Mina McGinn, Christopher Rabender, Ross Mikkelsen, Vasily Yakovlev
Archana Kamalakar, Brendan Tobin, Sundus Kaimari, M. Hope Robinson, Afra I. Toma, Timothy Cha, Samir Chihab, Irica Moriarity, Surabhi Gautam, Pallavi Bhattaram, Shelly Abramowicz, Hicham Drissi, Andrés J. García, Levi B. Wood, Steven L. Goudy
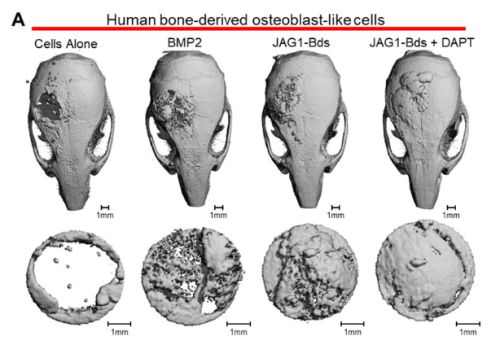
Jessica Honorato Ribeiro, Emre Etlioglu, Jasmine Buset, Ann Janssen, Hanne Puype, Lisa Berden, André Claude Mbouombouo Mfossa, Winnok H. De Vos, Vanessa Vermeirssen, Sarah Baatout, Nicholas Rajan, Roel Quintens
C Parikh, RA Glenn, Y Shi, K Chatterjee, EE Swanzey, S Singer, SC Do, Y Zhan, Y Furuta, M Tahiliani, E Apostolou, A Polyzos, R Koche, JG Mezey, T Vierbuchen, M Stadtfeld
| Plant development
Ying Xu, András Székely, Steffen Ostendorp, Saurabh Gupta, Melissa Tomkins, Lei Yang, Federico Apelt, Yan Zhao, Eleni Mavrothalassiti, Linda Wansing, Julia Kehr, Eleftheria Saplaoura, Friedrich Kragler
OsAAI1-OsMADS25 module orchestrates root morphogenesis by fine-tuning IAA in drought stressed rice
Ning Xu, Rui Luo, Qing Long, Jianmin Man, Jiaxi Yin, Haimin Liao, Meng Jiang
Nabila El Arbi, Sarah Muniz Nardeli, Jan Šimura, Karin Ljung, Markus Schmid
Javier Cabrera, Alvaro Sanchez-Corrionero, Angels de Luis Balaguer, Laura Serrano-Ron, Cristina del Barrio, Pilar Cubas, Pablo Perez-Garcia, Rosangela Sozzani, Miguel Moreno-Risueno
Histidine limitation causes alteration in the TOR network and plant development
Amandine Guérin, Caroline Levasseur, Aline Herger, Dominik Renggli, Alexandros Georgios Sotiropoulos, Gabor Kadler, Xiaoyu Hou, Myriam Schaufelberger, Christian Meyer, Thomas Wicker, Laurent Bigler, Christoph Ringli
Cell fate plasticity of xylem-pole-pericycle in Arabidopsis roots
Xin Wang, Lingling Ye, Jing Zhang, Charles W. Melnyk, Ari Pekka Mähönen
Martin William Battle, Scott Fraser Ewing, Cathryn Dickson, Joseph Obaje, Kristen N. Edgeworth, Rebecca Bindbeutel, Rea Antoniou Kourounioti, Dmitri A. Nusinow, Matthew Alan Jones
Dissecting the genetic regulation of lateral root development in tomato under salt stress
Maryam Rahmati Ishka, Hayley Sussman, Jiantao Zhao, Eric Craft, Li’ang Yu, Andrew Nelson, Miguel Pineros, Mark Tester, Dorota Kawa, Zhangjun Fei, Magdalena M. Julkowska
Aya Hanzawa, Arifa Ahamed Rahman, Abidur Rahman
An AINTEGUMENTA phospho-switch controls bilateral stem cell activity during secondary growth
Wei Xiao, Ling Yang, David Molina, Houming Chen, Shan Yu, Yingjing Miao, Dagmar Ripper, Shulin Deng, Martin Bayer, Bert De Rybel, Laura Ragni
Yolanda Durán-Medina, David Díaz-Ramírez, Humberto Herrera-Ubaldo, Maurizio Di Marzo, Andrea Gómez Felipe, J. Erik Cruz-Valderrama, Carlos A. Vázquez, Herenia Guerrero-Largo, Lucia Colombo, Ondrej Novak, Stefan de Folter, Nayelli Marsch-Martínez
Yan Wang, Seamus Kelley, Rodolfo Zentella, Jianhong Hu, Hua Wei, Lei Wang, Jeffrey Shabanowitz, Donald F. Hunt, Tai-ping Sun
Dorothy D. Sweet, Julian Cooper, Cory D. Hirsch, Candice N. Hirsch
J.-F. Trontin, M.D. Sow, A. Delaunay, I. Modesto, C. Teyssier, I. Reymond, F. Canlet, N. Boizot, C. Le Metté, A. Gibert, C. Chaparro, C. Daviaud, J. Tost, C. Miguel, M.-A. Lelu-Walter, S. Maury
In a nutshell: pistachio genome and kernel development
Jaclyn A. Adaskaveg, Chaehee Lee, Yiduo Wei, Fangyi Wang, Filipa S. Grilo, Saskia D. Mesquida-Pesci, Matthew Davis, Selina C. Wang, Giulia Marino, Louise Ferguson, Patrick J Brown, Georgia Drakakaki, Adela Mena-Morales, Annalisa Marchese, Antonio Giovino, Esaú Martínez, Francesco Paolo Marra, Lourdes Marchante Cuevas, Luigi Cattivelli, Paolo Bagnaresi, Pablo Carbonell-Bejerano, Grey Monroe, Barbara Blanco-Ulate
| Evo-devo
Brachiopod genome unveils the evolution of the BMP–Chordin network in bilaterian body patterning
Thomas D. Lewin, Keisuke Shimizu, Isabel Jiah-Yih Liao, Mu-En Chen, Kazuyoshi Endo, Noriyuki Satoh, Peter W. H. Holland, Yue Him Wong, Yi-Jyun Luo
The joint evolution of separate sexes and sexual dimorphism
Thomas Lesaffre, John R. Pannell, Charles Mullon
Eliana Pintus, Radim Kotrba, José Luis Ros-Santaella
Lepidopteran scale cells derive from sensory organ precursors through a canonical lineage
Ling S. Loh, Kyle A. DeMarr, Martina Tsimba, Christa Heryanto, Alejandro Berrio, Nipam H. Patel, Arnaud Martin, W. Owen McMillan, Gregory A. Wray, Joseph J. Hanly
Temporal dynamics of gene expression during metamorphosis in two distant Drosophila species
Aleksandra M Ozerova, Dina A. Kulikova, Michael B Evgen’ev, Mikhail S. Gelfand
Multiplexed transcriptomic analyses of the plant embryonic hourglass
Hao Wu, Ruqiang Zhang, Karl J. Niklas, Michael J. Scanlon
Lucy A. Winder, Jacob Hogger Gadsby, Eleanor Wellman, Joel L. Pick, Mirre J.P. Simons, Terry Burke
Convergent evolution of sex chromosomes in palms
H. Tessarotto, T. Beulé, E. Cherif, J. Orjuela, A. Lindstrom, A. Lemansour, M. Dahme, S. Santoni, J. Käfer, F. Aberlenc
Same trait, different genes: pelvic spine loss in three brook stickleback populations in Alberta
Jonathan A. Mee
Evolutionary bursts drive morphological novelty in the world’s largest skinks
Ian G. Brennan, David G. Chapple, J. Scott Keogh, Stephen Donnellan
Ecdysteroid-dependent molting in tardigrades
Shumpei Yamakawa, Andreas Hejnol
Cell Biology
N-Cadherin mediated cell rearrangements shape embryonic macrophage cluster
Jacob Hasenauer, Xiang Meng, Honor Scarborough, Jasmine A. Stanley-Ahmed, Darius Vasco Köster, Aparna Ratheesh
UNC-6/Netrin promotes both adhesion and directed growth within a single axon
Ev L. Nichols, Joo Lee, Kang Shen
Transcription templated assembly of the nucleolus in the C. elegans embryo
Nishant Kodan, Rabeya Hussaini, Stephanie C. Weber, Jane Kondev, Lishibanya Mohapatra
Systematic analysis of protein stability associated with species-specific developmental tempo
Mitsuhiro Matsuda, Henrik M. Hammarén, Jorge Lázaro, Mikhail M. Savitski, Miki Ebisuya
Maxime Goguet, Emilio J Vélez, Simon Schnebert, Karine Dias, Vincent Véron, Alexandra Depincé, Florian Beaumatin, Amaury Herpin, Iban Seiliez
Contribution of the neuron-specific ATP1A3 to embryonic spinal circuit emergencev
Sarah Dinvaut, Sophie Calvet, Jean-Christophe Comte, Raphael Gury, Olivier Pascual, Maelys André, Rosaria Ferrigno, Jérôme Honnorat, Frédéric Moret, Guillaume Marcy, Julien Falk, Valérie Castellani
Ryan M Finnerty, Daniel J Carulli, Akshata Hedge, Yanli Wang, Frimpong Boadu, Sarayut Winuthayanon, Jianlin Cheng, Wipawee Winuthayanon
Ziyun Yi, Qiu-xia Liang, Qian Zhou, Yang Lin, Qing-ren Meng, Jian Li, Yihua Lin, Chunhui Zhang, Heide Schatten, Jie Qiao, Qing-Yuan Sun
Yitong Xu, Anna Chao, Melissa Rinaldin, Alison Kickuth, Jan Brugués, Stefano Di Talia
PCM1 conveys centrosome asymmetry to polarized endosome dynamics in regulating daughter cell fate
Xiang Zhao, Yiqi Wang, Vincent Mouilleau, Ahmet Can Solak, Jason Garcia, Xingye Chen, Christopher J. Wilkinson, Loic Royer, Zhiqiang Dong, Su Guo
Identification of BiP as a temperature sensor mediating temperature-induced germline sex reversal
Jing Shi, Danli Sheng, Jie Guo, Fangyuan Zhou, Shaofeng Wu, Hongyun Tang
Kristin P. Kim, Christopher A. Lemmon
Cell type-specific regulation by different cytokinetic pathways in the early embryo
Caroline Q. Connors, Sophia L. Martin, Julien Dumont, Mimi Shirasu-Hiza, Julie C. Canman
Jeffrey Y Lee, Niles Huang, Tamsin J Samuels, Ilan Davis
Danielle F. Mello, Luiza Perez, Christina M. Bergemann, Katherine S. Morton, Ian T. Ryde, Joel N. Meyer
Modelling
The Molecular Basis of Differentiation Wave Activity in Embryogenesis
Bradly Alicea, Surosh Bastani, Natalie K. Gordon, Susan Crawford-Young, Richard Gordon
Statistical description of mobile oscillators in embryonic pattern formation
Koichiro Uriu, Luis G Morelli
Statistical description of mobile oscillators in embryonic pattern formation
Koichiro Uriu, Luis G. Morelli
Andreas Buttenschön, Shona Sinclair, Leah Edelstein-Keshet
Minimal cellular automaton model with heterogeneous cell sizes predicts epithelial colony growth
Steffen Lange, Jannik Schmied, Paul Willam, Anja Voss-Böhme
Tools & Resources
Zahra Elahi, Vanta Jameson, Magdaline Sakkas, Suzanne K Butcher, Justine D Mintern, Kristen J Radford, Christine A Wells
Interaction between gene expression and morphokinetic parameters in undisturbed human embryo culture
Hui Xiao, Adam Stevens, Helen L. Smith, Karolina Szczesna, Maria Keramari, Gregory Horne, Andras Dinnyes, Susan J. Kimber, Pietro Lio, Daniel R. Brison
Enhanced Plasmid-Based Transcriptional Activation in Developing Mouse Photoreceptors
Brendon M. Patierno, Mark M. Emerson
A Pluripotent Stem Cell Platform for in Vitro Systems Genetics Studies of Mouse Development
Rachel A. Glenn, Stephanie C. Do, Karthik Guruvayurappan, Emily K. Corrigan, Laura Santini, Daniel Medina-Cano, Sarah Singer, Hyein Cho, Jing Liu, Karl Broman, Anne Czechanski, Laura Reinholdt, Richard Koche, Yasuhide Furuta, Meik Kunz, Thomas Vierbuchen
Functional imaging of whole mouse embryonic development in utero
Jiejun Zhu, Dongming He, Mengzhu Sun, Hanming Zheng, Zihao Chen, Jin Yang, Chengqi Lin, Yun Stone Shi, Lei Sun, Zhihai Qiu
Ary Marsee, Arabela Ritchie, Adam Myszczyszyn, Shicheng Ye, Jung-Chin Chang, Arif Ibrahim Ardisasmita, Indi P Joore, Jose Castro-Alpízar, Sabine A Fuchs, Kerstin Schneeberger, Bart Spee
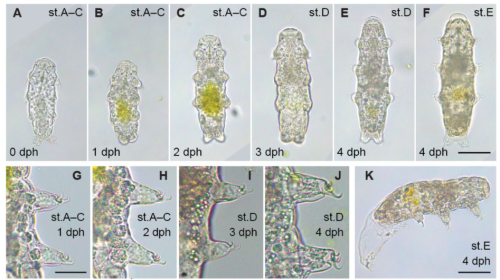
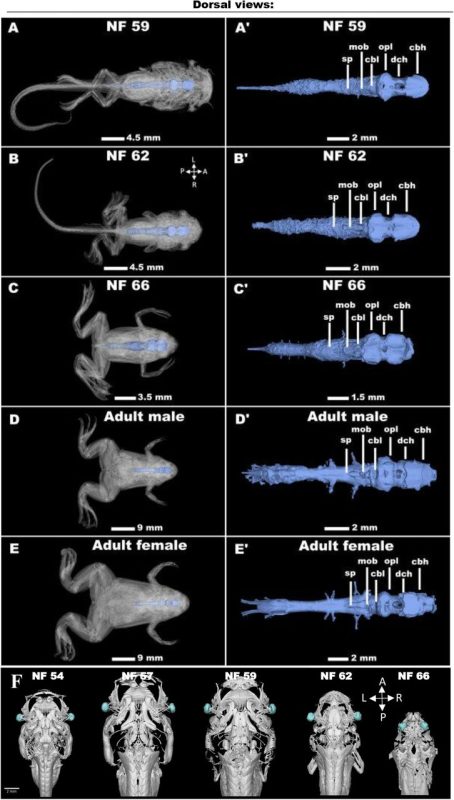
Unveiling Vertebrate Development Dynamics in Frog Xenopus laevis using Micro-CT Imaging
Laznovsky Jakub, Kavkova Michaela, Reis Alice, Robovska-Havelkova Pavla, Krivanek Jan, Zikmund Tomas, Kaiser Jozef, Buchtova Marcela, Harnos Jakub
Transcriptomic comparison of in vitro models of the human placenta
Samantha Lapehn, Sidharth Nair, Evan J Firsick, James MacDonald, Ciara Thoreson, James A Litch, Nicole R Bush, Leena Kadam, Sylvie Girard, Leslie Myatt, Bhagwat Prasad, Sheela Sathyanarayana, Alison G Paquette
Chemically induced cell plasticity enables the generation of high-fidelity embryo model
Huanhuan Li, Jiahui Huang, Wei Guan, Jinyi Wu, Haiping Luo, Litao Chang, Haiyong Zhao, Chuanxin Chen, Yake Gao, Jian Zhang, José C. R. Silva
Teresa Krammer, Elly M. Tanaka
An efficient method for immortalizing mouse embryonic fibroblasts
Srisathya Srinivasan, Hsin-Yi Henry Ho


 (No Ratings Yet)
(No Ratings Yet)
 (4 votes)
(4 votes)