“Unconventional” posts from #DevMeeting23: Mollusks
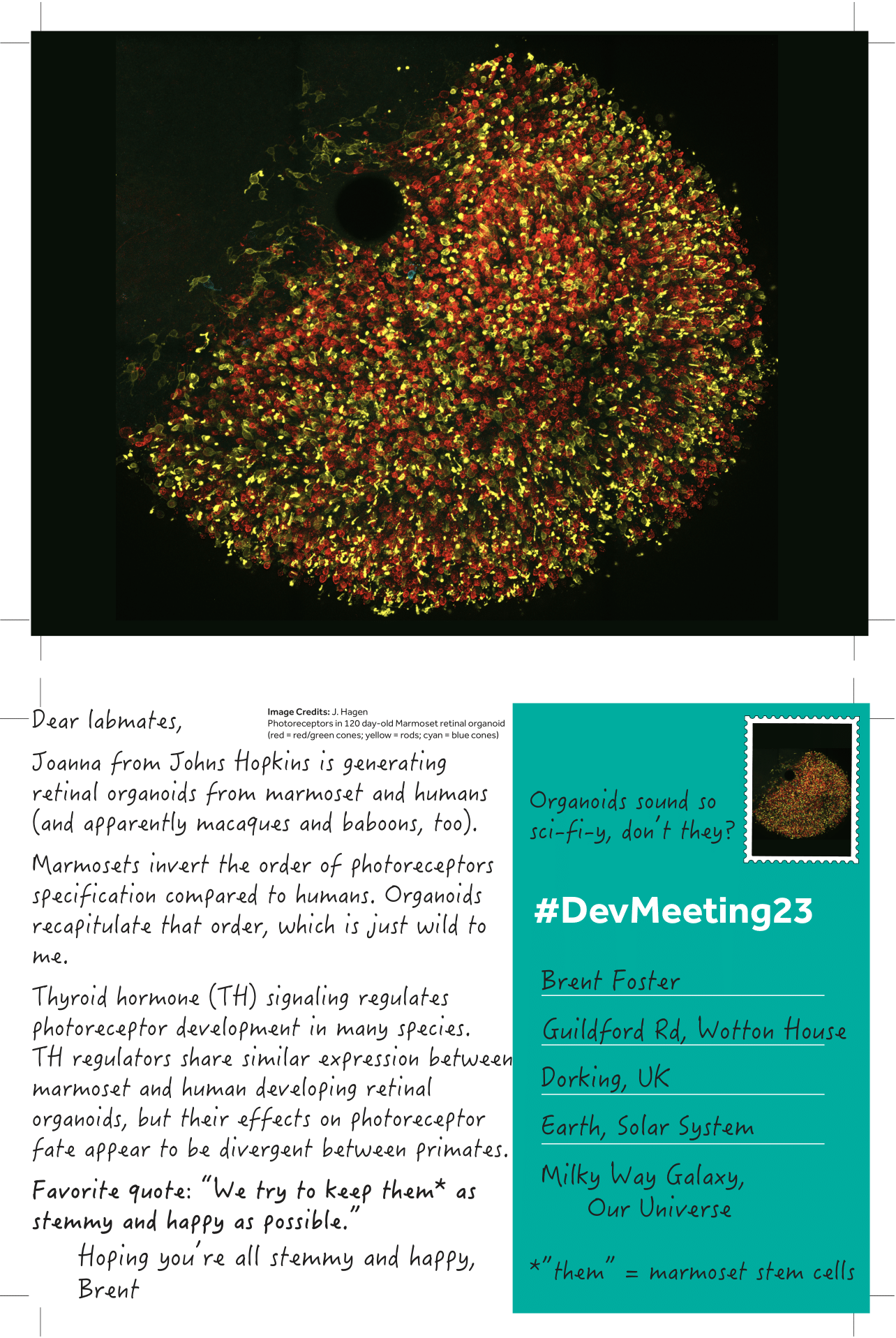
Posted by Brent Foster, on 20 September 2023
Posted by Brent Foster, on 20 September 2023
Posted by Brent Foster, on 20 September 2023
Posted by Brent Foster, on 20 September 2023

From Procko, Radin et al., 2022. “Dynamic calcium signals mediate the feeding response of the carnivorous sundew plant.” PNAS.
For this postcard, I’ve selected Ivan’s talk about calcium signaling in the sundew, but I wanted to give a shoutout to some of the conference’s other plant representatives. We’ve learned about
Posted by Brent Foster, on 20 September 2023
On Monday we learned a bit about early branching animals, especially the cnidarians. One poster mentioned ongoing work in ctenophores.


Note: I should point out that Nematostella wasn’t the only cnidarian mentioned at the conference. We’ve had representation from Hydra showing us how cell types may have evolved, as well as from Aiptasia teaching us about intracellular host-algal symbiosis.
Posted by the Node, on 19 September 2023
Have you ever had to interview someone for an article? What are the considerations before, during and after the interview?
A group of active members of the three community sites (the Node, FocalPlane and preLights) got together to discuss their experiences of interviewing people and shared a few tips and good practices.
We created a visual summary of the discussion on preLights. Check it out: How to conduct interviews – brainstorming session with members of the Node, preLights and FocalPlane. – preLights (biologists.com)
Posted by Brent Foster, on 19 September 2023

Posted by Alice Carstairs, on 19 September 2023
Help the NC3Rs fund the best new science that could replace, refine or reduce the use of animals in research.
Our panels are instrumental in supporting the development of new 3Rs models, promoting uptake and championing early career researchers and we are now inviting applications from talented senior researchers to become Panel members from January 2024. For this recruitment round, a wide range of expertise is sought after and applications are particularly welcome from individuals with industrial or interdisciplinary experience. Applications from women, those with a disability, and members of minority ethnic groups are especially encouraged.
For further information, including an application form, visit: https://nc3rs.org.uk/funding-panel-vacancies
The deadline for applications is 4pm (GMT) on Monday 20 November.
If you have any queries, or you would like further information, please email recruit@nc3rs.org.uk.

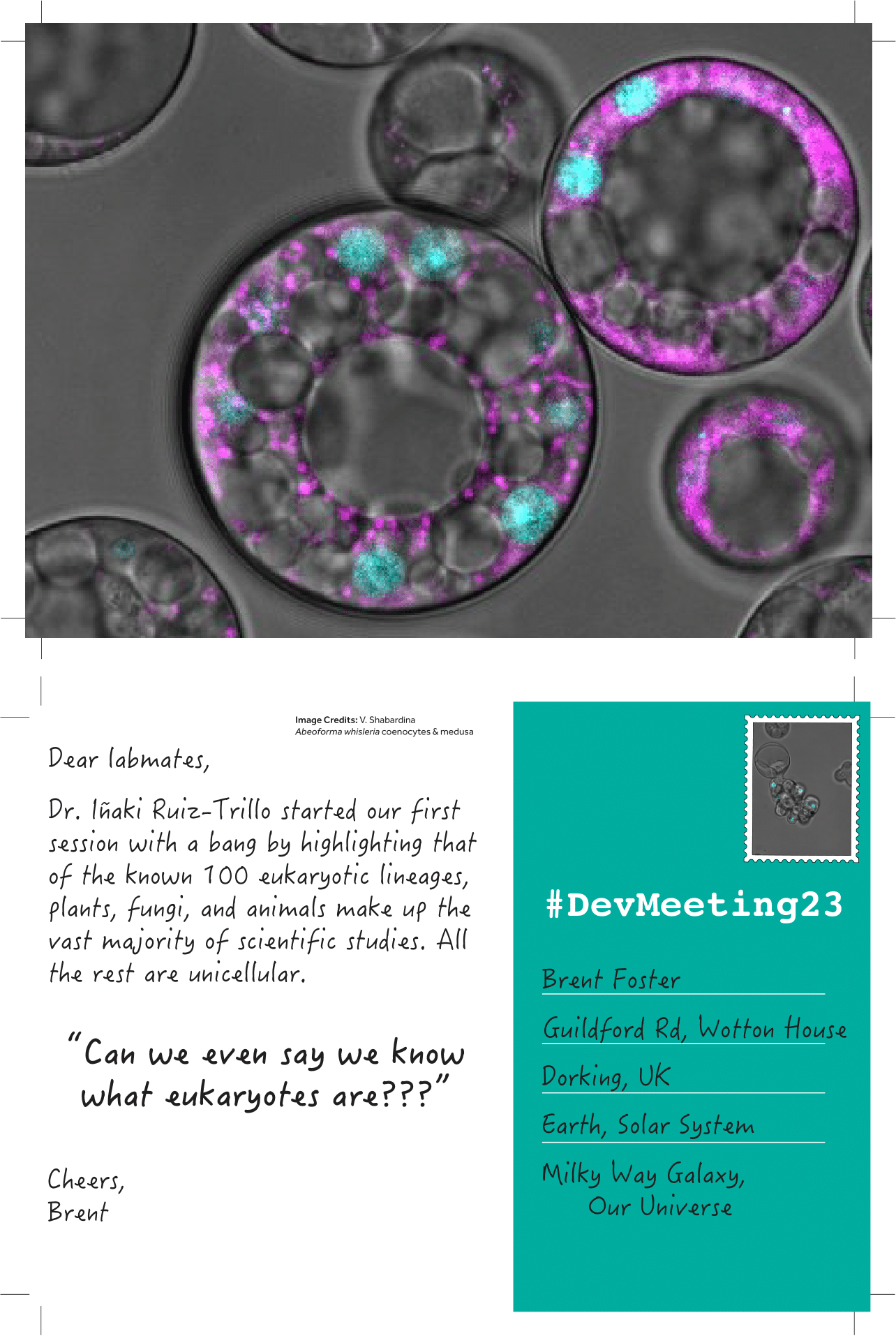
Posted by Brent Foster, on 18 September 2023
Introducing “unconventional posts,” a miniseries celebrating the “unconventional” experimental systems presented at #DevMeeting23. Each day I’ll upload handmade postcards spotlighting the breadth of unconventional systems shared in talks or posters and post it on The Node. Here are the first couple to get us started:

To read the other #DevMeeting23 postcards, visit this page: DevMeeting23 postcards Archives – the Node (biologists.com)
Posted by Dina Mikimoto, on 18 September 2023
When I was a child, I loved the movie “The Fifth Element”—the people working together with living beings from other planets, the space travel while you sleep, the queer clothes, the microwave that makes a fried chicken from a pressed tablet, and the restoration of a living being from a remaining piece. Years later, I am basically working on the last one: creating tissues from cells, building models of organs, and trying to use them to model healthy and diseased conditions. The field I am working in, tissue engineering and regenerative medicine, sounds as futuristic as the film is. And I often feel that people see this field of research in that way— as a science that emerged out of nowhere, in a kind of Frankesteinish and mad scientist way. Talking with people about this area of research and my work, I have heard different reactions, starting from fascination and ending with a question I was asked by one of my friends: “Is it even legal?”. So, I started wondering where this outlandish image was coming from. Is it the lack of awareness of its origin and its goals, or maybe the ethical controversies widely covered by the media, or is it the desire to have simple answers contradicting attempts to share complicated scientific progress?
The first attempts to replace and restore damaged organs
When I started working in this field, I didn’t look too much into history; I just loved the challenge of mixing different subjects and trying to unveil the biological processes by modeling organs outside of the human body. However, talking with people and seeing a lack of trust and disbelief that I was doing something useful or feasible inspired me to investigate the history of how tissue engineering and regenerative medicine came to be. I was struck by how logical its emergence was. The gist of this field is the desire to recreate such biological processes as development and disease outside of the human body and to replace or restore failed organs with artificially created ones. The interest in replacing some lost parts of the human body is not a modern idea, though. One of the oldest mentions that I found is the ancient text on medical and surgical practices from India written somewhere between one millennium BCE to the first centuries CE, which is called “Sushruta Samhita”. The text describes an autograft skin transplantation, which we would now probably call rhinoplasty. The procedure allowed restoration of the nose, which was tightly connected with the image of dignity in India and could be lost due to punishment or in wars1.
Another area with a long history of replacement attempts is dental implantology. Historical evidence suggests that as early as 7th century BC, Etruscans used golden wire and artificial teeth to replace loosened incisor teeth2. The first documented teeth transplantations, though, happened in Europe in the Middle Ages3. And titanium implants were discovered only in the 20th century.
The rapidly changing world of the 20th century
The 20th century brought the beginning of fast scientific progress and associated with it changes in the way we see the world. The work of Swiss surgeon Theodor Kocher (late 19th to 20th century), who perfected thyroid gland surgery and discovered its function, shaped how we see organ transplantation. His findings led to surgeons’ and physiologists’ interest in organ replacement, extensive experiments in animals, and attempts to use animal organs to replace failed organs in humans. Then Alex Carrel figured out how to reconnect the transplanted organ to the respective blood vessels in a host body (early 20th century). Gradually, the surgery skills reached the level when it became obvious that even when the surgery itself is done perfectly, organ transplantation does not lead to the intended results of restoring a fully functioning body. Through many theories, researchers then concluded that the main problem is probably connected with the immune system4. Starting around the 1950s, people gradually learned how to suppress the immune rejection of transplanted organs5 and how to transplant organs, first from living (kidneys) and then from deceased donors6.
The progress medicine and physiology saw in the 20th century is astonishing. Apart from the obvious positive influence of improved medical care and longer life expectancy, it brought a major philosophical shift. The development of organ transplantation marks the end of an era when the prevalent belief was that the human body is a whole thing and diseases are connected with disturbances of liquid flows inside it4. The human body becomes an assembly of organs. Questions like “Do organs hold a part of a host soul?” or “Will transplantation affect the soul of a patient?” captivate the minds of people. We still can see the remnants of these beliefs in art. Just last year, I watched a movie where the main heroine receives a heart transplant and falls in love with a stranger, only to discover that it was the soul of the heart donor living inside her. Everything changes: the way people see themselves, the way people define death, and the way people see each other. It brings the necessity to develop ethical and legal rules for organ donation and transplantation, for experiments on human beings and animals. A lot of questions only become visible with time, like the differences in legal regulations of organ transplantation in different countries resulting in medical tourism.
In parallel with organ transplantation, techniques for growing cells outside the body were developed. First, the physiological solutions, like Ringer’s solution, which allowed keeping organs alive outside the animal body for several hours or days (late 19th century). Then the cells are successfully cultured in vitro by Margaret Reed Lewis (early 20th century), the standard culture medium recipe is developed, and the standardized cell lines appear7. Science is constantly changing people’s views on what is possible. Advances in cell culture bring the question of the possibility of recreating organs outside of the human body. And here we are, seeing the beginning of tissue engineering and regenerative medicine at the end of the 20th century.
The role of communication in scientific progress
While reading about the history of the tissue engineering and regenerative medicine field, I noticed that there wasn’t a lot of information about how these discoveries were accepted by public. Just imagine being born and raised with the idea that your body is the vessel of a soul and then being told that, actually, it is just like a clock – a mechanism with many replaceable parts. I remember a similar experience from my childhood. In primary school, I was told that you should always subtract a smaller number from a bigger one; you can’t do it vice versa. And then, in middle school, I learned that you actually can, and then you will get a negative number. Even though that was the way everyone learned mathematics, I felt betrayed. Why was it necessary to conceal the truth about the possibility of subtracting whatever you want from whatever you want? And what else is concealed until the time to know comes?
I guess part of the answer lies in how science was communicated throughout history. For a long time, science was mainly practiced by the privileged social classes, and communication mostly happened in a kind of top-down direction, where scientists or their benefactors showcased or shared only the things they wanted to share. Only in the 20th and 21st centuries science communication started to be seen as a way to allow people to make informed decisions and even influence the research direction when it concerns the everyday life of society 8. Part of the answer lies in the way science is taught as a subject with straightforward results, skipping a part where scientists were not sure or had conflicting ideas. As a result, when this process is shared, and some facts scientists believe to be true turn out to be wrong, instead of natural progression, it looks like not trustworthy. But also when reading stories like “The Immortal Life of Henrietta Lacks”, documented by Rebecca Skloot, or a recent article about using the CRISPR technique for editing human embryos by Dana Goodyear9, the thing that bothers me is often too superficial communication between scientists themselves. Too often connections are just networking when you briefly listen to 10 minutes of results that took a couple of years to get and then congratulate each other with a newly published article without looking too much into the details. Too rarely it is a meaningful connection when you are interested in another person’s work and ready for in-depth discussions.
I feel that lately, the academic structure is pushing for faster, bigger, better. It needs simple narratives and fast results. The never-ending competition brings the over-focus on your own work; researchers are constantly searching for their own niche, something to be at least a little bit different from others. It sometimes feels that instead of solving the problem, it is important to solve it differently from others. As a result, we have so much literature in our own domain that we hardly have time to follow other fields of science; we are so focused on our own research that hardly have time to listen and understand others. We are constantly networking and collaborating, but it feels that we are as isolated from each other as we can possibly be. Lately, I started asking myself questions. How often do you read papers that are not from your field? How often do you go for an adventure and read on completely unrelated topics? When was the last time you attended an interdisciplinary conference? And I mean truly interdisciplinary, not around the topic you work on, but rather an event where different people talk about the universe, climate change, medicine, and literature in one place. We all want to be heard, but how often are we the ones listening?
I first came up with the question of why people think of tissue engineering and regenerative medicine as a strange science because I felt misunderstood, but the longer I think about it, the more I think that it is a reflection of how the academic system is currently working. And maybe this is one of the reasons I decided to try science communication – to become the one who listens.
Further reading:
1. Saraf S. Sushruta: Rhinoplasty in 600 B.C. The Internet Journal of Plastic Surgery 2006; 3.https://ispub.com/IJPS/3/2/7839 (accessed 8 Sep2023).
2. Donaldson JA. The use of gold in dentistry: An historical overview. Part I. Gold Bull 1980; 13: 117–124.
3. Pasqualini U, Pasqualini ME. THE HISTORY OF IMPLANTOLOGY. In: Treatise of Implant Dentistry: The Italian Tribute to Modern Implantology. Ariesdue, 2009https://www.ncbi.nlm.nih.gov/books/NBK409631/ (accessed 18 Aug2023).
4. Schlich T. The origins of organ transplantation. The Lancet 2011; 378: 1372–1373.
5. Allison AC. Immunosuppressive drugs: the first 50 years and a glance forward. Immunopharmacology 2000; 47: 63–83.
6. Nordham KD, Ninokawa S. The history of organ transplantation. Proc (Bayl Univ Med Cent); 35: 124–128.
7. Yao T, Asayama Y. Animal‐cell culture media: History, characteristics, and current issues. Reprod Med Biol 2017; 16: 99–117.
8. Nielsen KH. Histories of Science Communication. Histories 2022; 2: 334–340.
9. Goodyear D. The Transformative, Alarming Power of Gene Editing. The New Yorker 2023.https://www.newyorker.com/magazine/2023/09/11/the-transformative-alarming-power-of-gene-editing (accessed 7 Sep2023).
Posted by the Node, on 18 September 2023
Development’s journal meeting, Unconventional and Emerging Experimental Organisms in Cell and Developmental Biology, took place between 17-20 September at the beautiful Wotton House in Surrey, UK. We’re delighted to be able to live-stream one session of this meeting to the broader community.
The event featured two fantastic talks, followed by a panel discussion session.
| 16:00 (BST) | Ehab Abouheif (McGill University) ‘Ants, eco-evo-devo, and the evolution of biological complexity’ |
| 16:30 | Kim Cooper (UC San Diego) ‘Evolution of extreme skeletal proportion in bipedal jerboas’ |
| 17:00 | Why we need to study unconventional models in the light of climate and biodiversity challenges Chair: Cassandra Extavour Panel: Ehab Abouheif, Kim Cooper, Michael Raissig, Iñaki Ruiz-Trillo |
| 18:00 | Close |